More Information
Submitted: March 19, 2025 | Approved: April 02, 2025 | Published: April 02, 2025
How to cite this article: Kalita J, Kumar D, Gutti NB, Gupta SK, Mishra1 A, Singh V. A Comparative Study of Metoprolol and Amlodipine on Mortality, Disability and Complication in Acute Stroke. J Neurosci Neurol Disord. 2025; 9(1): 039-045. Available from:
https://dx.doi.org/10.29328/journal.jnnd.1001108
DOI: 10.29328/journal.jnnd.1001108
Copyright License: © 2025 Kalita J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Intracerebral hemorrhage; Infarction; Beta-blocker; Calcium channel blocker; Outcome
Introduction: ICH: Intracerebral Hemorrhage; IS: Ischemic Stroke; GI: Gastrointestinal hemorrhage; RCT: Randomized Controlled Trial; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; GCS: Glasgow Coma Scale; NIHSS: National Institute of Health Stroke Scale; mRS: modified Rankin Scale; SIRS: Systemic Inflammation Response Syndrome; ASPECTS: Alberta Stroke Program Early CT score
A Comparative Study of Metoprolol and Amlodipine on Mortality, Disability and Complication in Acute Stroke
Jayantee Kalita1*, Dhiraj Kumar1, Nagendra B Gutti1, Sandeep K Gupta1, Anadi Mishra1 and Vivek Singh2
1Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow, Uttar Pradesh-226014, India
2Department of Radio diagnosis Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow, Uttar Pradesh-226014, India
*Address for Correspondence: Jayantee Kalita, Professor, Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Raebareli Road, Lucknow, Uttar Pradesh 226014, India, Email: [email protected]; [email protected]
Stress in acute stroke may increase mortality and complications, but there is a paucity of information on the efficacy of beta blockers over other anti-hypertensive. To report efficacy of metoprolol over amlodipine in reducing mortality, disability and infections in acute stroke. CT/MRI confirmed stroke patients within 3 days of onset were included whose age was 18 to 75 years. Patients with secondary intracerebral hemorrhage, organ failure, pregnancy, malignancy, and immunosuppressant or on beta-blocker/amlodipine were excluded. Stroke risk factors, Glasgow Coma Scale (GCS) score, National Institute of Health Stroke Scale (NIHSS) score and CT/MRI findings were noted. Patients with a blood pressure of > 160/90 mm of Hg were randomized using 1:1 randomization to metoprolol (25 mg on day 1, 50 mg if BP is not controlled) or amlodipine (2.5 mg on day 1, then 5 mg then 10 mg on, subsequent days if BP is not controlled). Other standard treatment was continued. The primary outcome was mortality at 1 month; secondary outcomes included were in-hospital gastrointestinal hemorrhage, pneumonia, sepsis and 3 months functional outcome based on modified Rankin Scale (mRS). Side effects were noted. 18 (14.4%) patients died; 6 (9.7%) in metoprolol and 12 (19%) in amlodipine (p = 0.20) group. At 3-months, 66 patients had good outcome; 45 (80.4%) in metoprolol and 21 (43.3%) in amlodipine group (p < 0.001). The other secondary outcomes were comparable between the two groups. Metoprolol was withdrawn in 6 patients due to bradycardia, and amlodipine in 5 due to hypotension and in 1 due to allergic reaction. Metoprolol is associated with improved functional outcomes in acute stroke compared to amlodipine.
Stroke is the second leading cause of death and the third leading cause of death and disability combined in 2019 [1]. Hypertension, diabetes, hyperlipidemia, obesity, sedentary life style and smoking are modifiable risk factors of stroke [2,3]. About 90% of stroke can be attributed to these modifiable risk factors, and 80% of recurrent stroke can be prevented by optimal control of these risk factors [1]. The mortality of stroke can be reduced by optimal management of blood pressure, raised intracranial pressure, blood glucose, electrolytes, temperature and preventing infection [4,5]. Animal and human studies have shown increased sympathetic activity during acute stage leading to reactive hypertension and cardiac arrhythmia [6]. Autonomic dysfunction and sympathetic overstimulation may have a role in stroke induced immuno-suppression, making the patient susceptible for infection [7,8]. The pathological sympathetic activation with a surge of catecholamines occurs in acute phase of stroke that is the initial stage, that leads to oxidative stress expression of pro-inflammatory cytokines and apoptotic markers resulting in increased brain edema and neuronal death [9]. Hypothetically, sympathetic blockers may reduce some of these biomarkers, and may have a mortality and disability benefits. Metoprolol reduces neuro-excitotoxicity, has anti-apoptotic actions, maintains blood-brain barrier integrity, and modulates neuroinflammation. In animal model of stroke, beta-blocker has resulted in reversal of catecholamine induced immune-suppression as evidence by reduction of oxidative stress and inflammatory cytokines [10,11]. In National Acute Stroke Israeli Study (NASIS) registry, patients on beta-blocker developing stroke had a better outcome, compared to those without beta blocker [12]. A randomized controlled study has also reported similar results [13]. In the retrospective data analysis, few studies have shown reduction in mortality [14], infection and pneumonia [15], and others did not find significant benefit [16]. There is no head on RCT comparing the effect of metoprolol and amlodipine on mortality and disability of stroke. In this randomized controlled trial, we compare the effect of metoprolol and amlodipine in reducing mortality and disability in acute stroke. We also compare in-hospital gastro-intestinal hemorrhage, pneumonia and sepsis between the two groups.
This is an investigator-initiated trial, open-labelled randomized control trial. The study protocol was designed by the lead author (JK). The protocol was approved by the Institute Ethics Committee (Ethics No: 2022-20-IMP-125 08/07/2022), SGPGI, Lucknow, and registered in Clinical Trial Registry-India (CTRI link: 2022/07/043879). Consent was obtained from patients or their first-degree relatives.
Inclusion criteria
Consecutive CT/MRI proven stroke (ischemic and hemorrhagic) patients admitted to our hospital within 3 days of ictus were screened for possible inclusion in the trial.
Exclusion criteria
Patients with stroke due to arteriovenous malformation, aneurysm, coagulopathy, bleeding disorders, tumor, trauma or vasculitis were excluded. Patients with cardiac, hepatic or renal failure, pregnancy, malignancy, immune-suppression, organ transplantation, malignancy, intracranial or systemic infection, prior use of β and α-blockers, and those below 18 years and above 75 years were excluded. Patients with a history of allergy to amlodipine and metoprolol, and those with bradyarrhythmia and hypotension were also excluded. Stroke patients with history of hypertension but normal blood pressure at presentation were also excluded.
Evaluation
A detailed clinical history including stroke risk factors was enquired, and clinical examination was done. Consciousness was assessed by Glasgow Coma Scale (GCS) and severity of stroke by National Institute of Health Stroke Scale (NIHSS). Clinical evidences of raised intracranial pressure and herniation such as hyperventilation, pupillary asymmetry and extensor posturing were noted. Cranial nerve palsy, muscle tone, power and tendon reflexes were recorded. Sensations and co-ordination were tested only in conscious, co-operative patients. Patients with infections were treated with antibiotics, and those with seizure received anti- seizure medications. All the patients received treatment of underlying stroke risk factors along with supportive care.
Investigations
Hemoglobin, blood counts, blood glucose, serum creatinine, electrolytes and lipid profile were measured. Activated partial thromboplastin time, chest radiograph, and electrocardiogram were done. The markers of systemic inflammation response syndrome (SIRS) were noted [17], cranial CT scan and or MRI were done at admission and the nature of stroke (intracerebral hemorrhage or infarction) was noted. The size of ICH volume was assessed by A x B x C x1/2 (A is the largest diameter of hematoma, B is the diameter perpendicular to A, and C in the number slices of hematoma multiplied by slice thickness). The hematoma size was calculated as small (< 20 ml), medium (20 – 40 ml) and Large (> 40 ml) [18]. The infarct size was calculated using Alberta Stroke Program Early CT score (ASPECTS) [19]. Midline shift was measured.
Treatment
Ischemic stroke patients within 4.5 hours with a NIHSS score of 6-25 were thrombolyzed using Tenecteplase (0.25 mg/kg IV) followed by standard treatment. All the patients received supportive care.
Randomization
The patients with a blood pressure of ≥ 160/100 mm Hg were randomized to metoprolol or amlodipine using computer based 1:1 randomization method. The starting dose of metoprolol was 25 mg, which increased to 50 mg depending on the blood pressure and heart rate. Similarly, amlodipine 2.5 mg was prescribed, which increased to 5 mg, then to 10 mg. The time period for each arm for dose escalation was done at 24 hours interval. Patients were managed in intensive care with regular monitoring of blood pressure, ECG, and oxygen saturation at 4-hour interval or earlier if patient was unstable. The target blood pressure was ≤ 160/90 in the initial 4 weeks, and ≤ 140/90 after 4 weeks. Hydrochlorothiazide was added, if blood pressure is not controlled.
The patients with thrombotic stroke received dual antiplatelets (aspirin 150 mg and clopidogrel 75 mg daily) and embolic stroke patients received anticoagulant. Anti-edema treatment (100 ml of 20% mannitol) was prescribed depending on the clinical evidence of raised intracranial pressure.
Outcome measures
In-hospital death and outcomes at 3 months were assessed using modified Rankin Scale (mRS). Occurrence of pneumonia, septicemia and SIRS during hospital stay was noted.
Primary outcome: Death at one month
Secondary outcomes: The secondary outcomes included gastric hemorrhage, SIRS, pneumonia and septicemia within hospital stay, and 3 months functional outcome. We have used standard laid down criteria for defining SIRS, pneumonia and septicemia [17,20-22]. The functional outcome was assessed using mRS as good (mRS 0-2) and poor (mRS 3-5) [23].
Sample size calculation: In an earlier report, the mortality of stroke patient in beta blocker group was 11.4% and non- beta blocker 37.3% [24]. At a minimum two-sided 95% confidence interval (CI) and 90% power of study, the estimated sample in each group was 56. A total of 120 patients were expected to randomize considering 5% loss to follow up. The sample size was calculated using G* power version 3.1.9.7.
Statistical analysis
The distribution of continuous variable was evaluated using Wilk-Shapiro test. The baseline categorical characteristics of clinical, radiological and laboratory were compared by chi square test, continuous normally distributed data using independent-t test and skewed continuous variables using Mann-Whitney U test. Intention to treat analysis of primary outcome was done. Both primary and secondary outcomes between the two treatment arms were compared using chi-square test. Kaplan-Meier analysis was used to evaluate the effect of the two treatments on mortality. Predictors of death and 3-months functional outcome were evaluated using univariate followed by multivariate analysis adjusting the variables significantly associated with death and good outcome on univariate analysis. Statistical analysis was done using SPSS software, and a variable with a two-sided p - value of < 0.05 in the statistical analysis was considered significance.
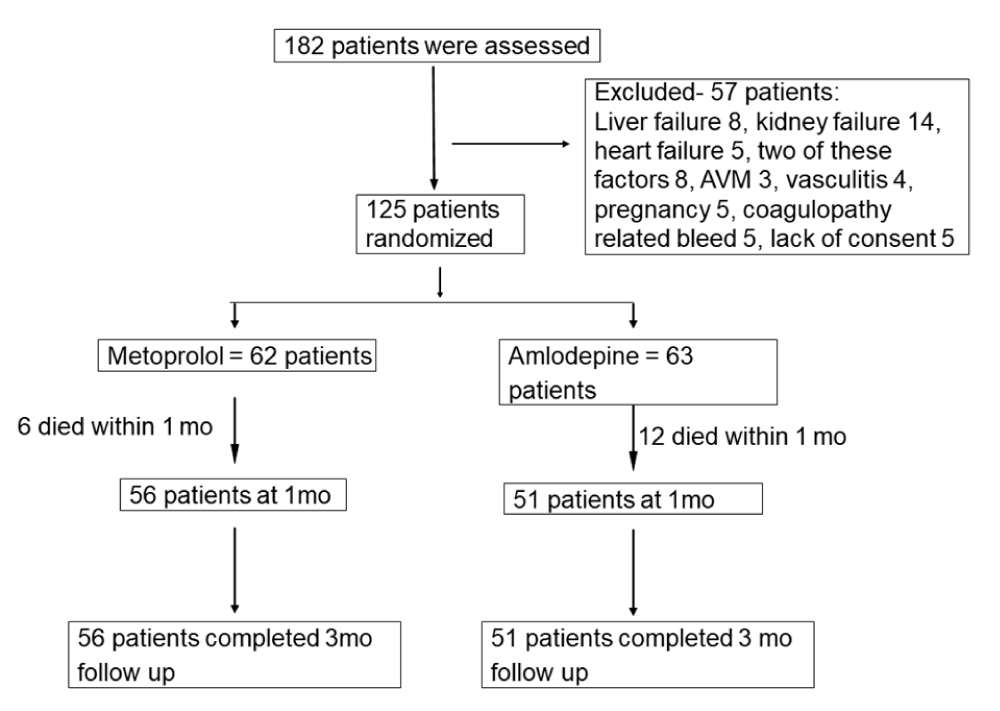
182 patients with stroke were admitted within 3 days of their illness; 57 patients were excluded due to associated liver, kidney or heart failure, arteriovenous malformation or coagulopathy related bleeding, vasculitis, pregnancy and malignancy. Therefore, this study is based on 125 patients (Figure 1). Their median age was 62 (range 29-88) years, and 47 (31%) were females. 119 patients had stroke risk factors. 62 patients were randomized to metoprolol and 63 to amlodipine. Their baseline characteristics were comparable except medium size stroke was more frequent in metoprolol group. The details are presented in Table 1.
Figure 1: Flow chart showing the number patients at different phases AVM: arteriovenous malformation
| Table 1: Baseline characteristics of patients in amlodipine and metoprolol groups. | ||||
| Parameters | Total (n = 125) |
Amlodipine (n = 63) |
Metoprolol (n = 62) |
p - value |
| Age (years) | 60.89 ± 11.22 | 59.2 ± 11.40 | 62.5 ± 10.8 | 0.10 |
| Gender (Male) | 78(62.4%) | 42(66.7%) | 36(58.1%) | 0.36 |
| Hypertension | 109(87.2%) | 51(80.90%) | 58(93.54%) | 0.27 |
| Diabetes mellitus | 33(26.4%) | 13 (20.6%) | 20(32.3%) | 0.13 |
| Heart disease: | 0.91 | |||
| CAD | 27(21.6%) | 12(19%) | 15(24.2%) | |
| RHD | 3(2.4%) | 2(3.2%) | 1(4%) | |
| Non Val. AF | 8(6.4%) | 4(6.3%) | 4(6.5%) | |
| Smoking | 21(16.8%) | 11(17.5%) | 10(16.1%) | 0.84 |
| LDL (mg/dl) | 104.57 ± 49.70 | 107.04 ± 52.16 | 102.06 ± 47.36 | 0.58 |
| NIHSS score | 17.14 ± 7.47 | 16.8 ± 8.1 | 17.5 ± 6.8 | 0.64 |
| GCS score | 11.38 ± 3.15 | 13.1 ± 1.7 | 13 ± 2 | 0.68 |
| Seizure | 21(16.8%) | 12(19%) | 9(14.5%) | 0.50 |
| Type of stroke | 0.13 | |||
| Ischemic | 42(33.6%) | 17(26.98%) | 25(40.32%) | |
| ICH | 83(66.4%) | 46(73.01%) | 37(59.68%) | |
| Size of stroke | 0.02 | |||
| Large | 35(28%) | 20(31.7%) | 15(24.2%) | |
| Medium | 49(39.2%) | 17(27%) | 32(51.6%) | |
| Small | 41(32.8%) | 26(41.3%) | 15(24.2%) | |
| ML shift (cm) | 0.42 ± 0.52 | 0.48 ± 0.58 | 0.57 ± 1.79 | 0.71 |
| Sr. Creatinine (mg/dl) | 1.37 ± 0.83 | 1.4 ± 0.8 | 1.3 ± 0.9 | 0.78 |
| Sodium (mmol/L) | 138.32 ± 6.99 | 138.8 ± 7.7 | 137.7 ± 6.3 | 0.37 |
| Potassium (mmol/L) | 3.9 ± 0.53 | 3.9 ± 0.6 | 3.9 ± 0.5 | 0.42 |
| SIRS at admission | 92(73.6%) | 48(76.2%) | 44(71.0%) | 0.55 |
| CAD: Coronary Artery Disease; RHD: Rheumatic Heart Disease; Non val. AF: Non Valvular Atrial Fibrillations; LDL: Low Density Lipoprotein; NIHSS: National Institute of Health Stroke Scale; GCS: Glasgow Coma Scale; ICH: Intracranial Hemorrhage; ML shift: Midline shift; SIRS: Systemic Inflammatory Response Syndrome | ||||
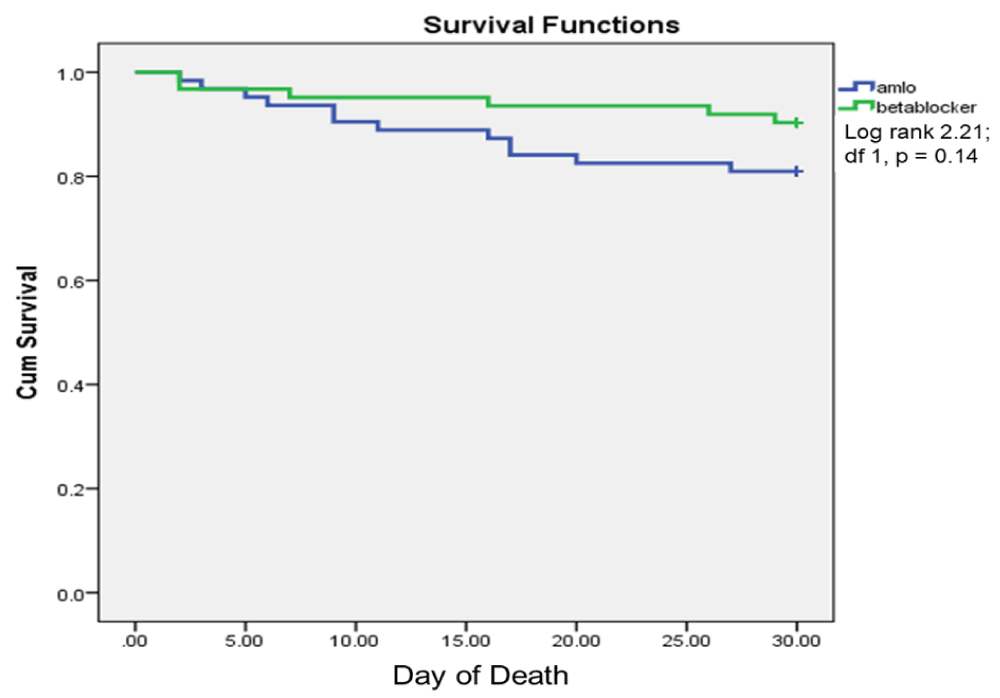
Primary outcome: 18 (14.4%) patients died at one month; 6 (9.7%) in metoprolol and 12 (19%) in amlodipine group (p = 0.20). Ten patients died within 2 weeks of admission, and eight died between weeks 2–4. On Kaplan-Meier analysis, the occurrence of death was not significantly different between metoprolol and amlodipine (log rank 0.94, chi square p = 0.33; Figure 2). In subgroup analysis, mortality in ischemic stroke (2 vs. 3, p = 0.64) and ICH (4 vs. 9 p = 0.36) were not significantly different between metoprolol and amlodipine group.
Figure 2: Kaplan-Meier survival analysis showing day of death within one months in amlodipine (Amlo) and metoprolol (Meto) groups.
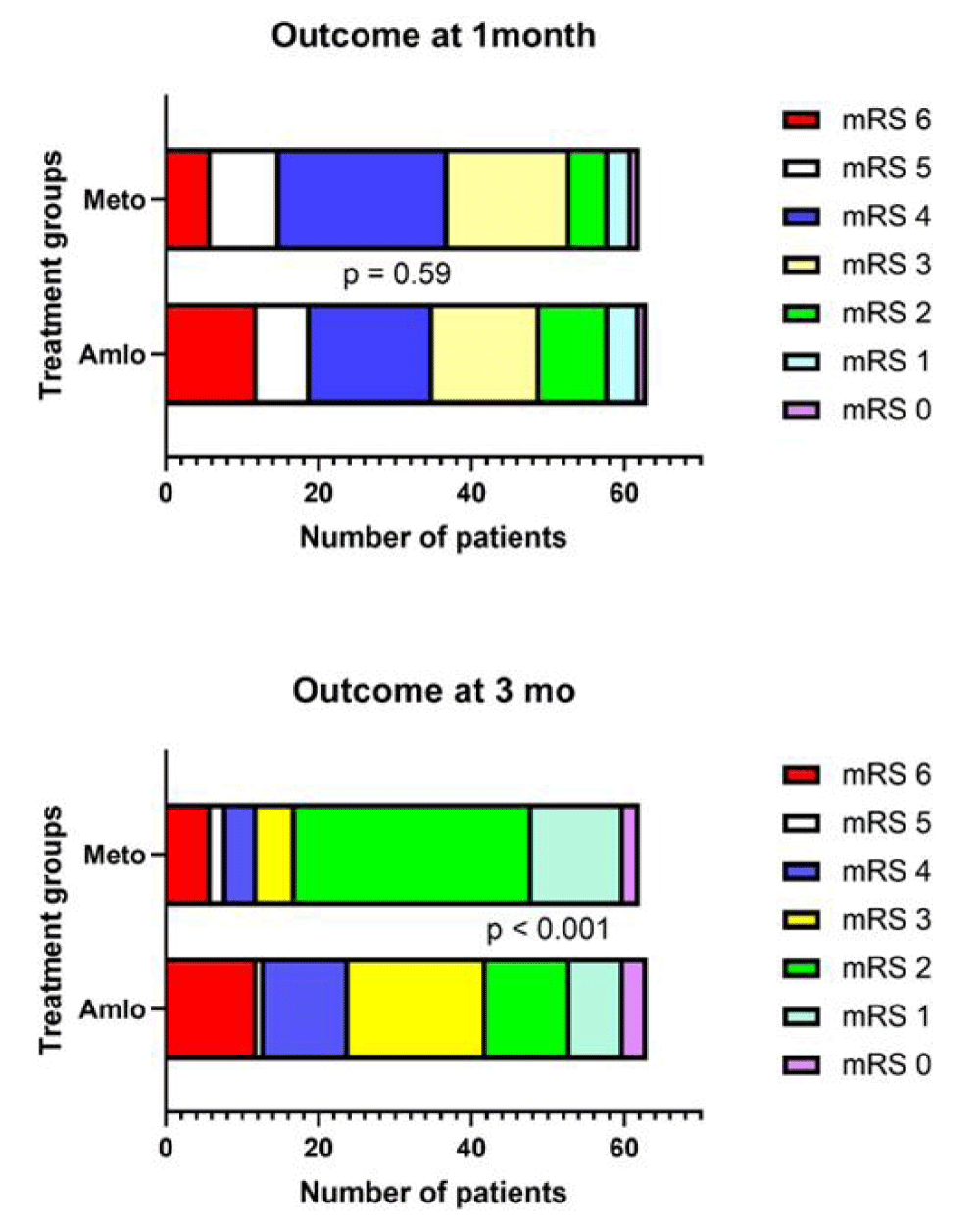
Secondary outcome: None of the patients died between one and 3 months of follow up. In metoprolol group, 45 (80.4%) patients had good outcome at 3 months, whereas only 21 (43.3%) patients in amlodipine group had good outcome (p < 0.001; Figure 3).
Figure 3: Bar diagram shows modified Rankin Scale (mRS) score at one month and 3-months in amlodipine (Amlo) and metoprolol (Meto) groups.
The other secondary outcome measures including GI hemorrhage, pneumonia and sepsis were not significantly different in metoprolol and amlodipine group (Table 2).
| Table 2 Outcome of stroke patients in amlodipine and metoprolol groups. | |||||
| Outcome parameters | Total (n = 125) |
Amlodipine (n = 63) |
Metoprolol (n = 62) | p - value | |
| Primary outcome | Death at 1 month | 18(14.4%) | 12(19%) | 6(9.7%) | 0.20 |
| Secondary outcomes | Pneumonia | 32(25.6%) | 16(25.4%) | 16(25.8%) | 1.00 |
| Sepsis | 10(8%) | 6(9.5%) | 4(6.5%) | 0.74 | |
| SIRS on day 7 | 74(59.2%) | 34(59.6%) | 40(65.6%) | 0.57 | |
| SIRS on day 15 | 35(28.0%) | 17(32.1%) | 18(29.5%) | 0.84 | |
| GI Hemorrhage | 20(16.0%) | 10(15.9%) | 10(16.1%) | 1.00 | |
| Outcome at 3-months | < 0.001 | ||||
| Good | 66(52.8%) | 21(41.2%) | 45(80.4%) | ||
| Poor | 41(32.8%) | 30(58.8%) | 11(19.6%) | ||
| Death | 18(14.4%) | 12(19%) | 6(9.7%) | ||
| GI: Gastrointestinal; SIRS: Systemic Inflammatory Response syndrome. | |||||
These outcome measures in ICH and IS were also comparable between the metoprolol and amlodipine groups (Supplementary Tables)Supplementary-Tables
Adverse events: 13 patients had adverse events; 6 in metoprolol and 7 in amlodipine group (p = 1.00). 6 patients were withdrawn from metoprolol because of bradycardia, and 5 due to hypotension and one due to drug allergy from amlodipine. The details are presented in Table 3.
| Table 3: Adverse events. | |||
| Adverse effects | Amlodipine (n = 63) | Metoprolol (n = 62) | p - value |
| Total | 7(11.1%) | 6(9.7%) | 1.00 |
| Bradycardia | 1(1.6%) | 6(9.7%) | < 0.05 |
| Allergy | 1(1.6%) | 0(0%) | 1.00 |
| Hypotension | 5(7.9%) | 0(0%) | < 0.05 |
Predictors of death and good outcome
On univariate analysis, death was associated with GCS at admission (p < 0.001), midline shift (p = 0.02), raised Trop I at admission (p = 0.04), size of stroke (p < 0.001), NIHSS (p < 0.001) and SIRS at admission (p = 0.04) (Supplementary table 2). On multivariate analysis after adjusting these variables, the independent predictor of death was GCS score at admission (Adjusted Odds ratio 0.67; 95% confidence interval 0.49 - 0.90; p = 0.008).
Predictors of good outcome on univariate analysis were use of beta blocker (p < 0.001), SIRS at day 7 (p = 0.01) and at day 15 (p < 0.001) (Supplementary Table 3). On multivariate analysis, the independent predictors of good outcome are use of beta blocker (AOR 13.37, 95% CI 3.97 - 45.00, p < 0.001) and SIRS at day 15 (AOR 0.14, 0.03 - 0.55, p = 0.005).
In this study, the patients on metoprolol had insignificant mortality benefit at 1 month and surviving patients more frequently achieved good functional recovery at 3 months. Pneumonia, SIRS, sepsis and GI hemorrhage were comparable between the two treatment arms. This randomized controlled trial has comprehensively evaluated the effect of metoprolol in mortality and functional outcome compared to amlodipine. Only 2 RCTs evaluated the role of beta blocker in acute stroke [13,25]. Barer, et al., included 302 conscious hemispheric stroke patients and randomized to atenolol, propranolol or placebo for 3 weeks. Deaths occurred more frequently in the beta-blocker group, and the 6-month functional outcome was not significantly different. 60 patients received beta blocker prior to stroke, and their outcome was however better [13]. In Controlling Hypertension and Hypotension Immediately Post-Stroke (CHHIPS) study, 179 patients were randomized to lisinopril, labetalol or placebo. At 2 weeks, there was no difference in mortality and dependency. At 3 months, active treatment group had lower mortality than placebo. 96 patients had severe adverse effect, which were comparable between the groups. Six patients required withdrawal from labetalol, 8 from lisinopril and 4 from placebo group (CHHIPS study) [25]. In a retrospective analysis of 841 patients, 10.6% patients received beta blocker during hospitalization. Death occurred less frequently in beta blocker group compared to non-beta blocker group (6.8% versus 19%; p < 0.01). Use of beta-blocker predicted survival (AHR 0.37, 95% CI 0·16-0.84) after adjustment of age, stroke severity, fasting blood sugar, cholesterol and pneumonia [14]. Retrospective studies have shown conflicting results, some have shown outcome benefit [15,26], and others did not [27-29]. Balla, et al., have done a meta-analysis to evaluate effect of beta- blocker on stroke outcome including 20 studies; of which only 2 were randomized trials. Beta blocker did not reveal benefit in mortality, functional outcome and infection prevention. These studies were heterogeneous and had moderate bias [16]. Our study did not show a statistically significant mortality benefit, although the 3 months functional outcome was better in metoprolol group.
We have found comparable frequency of pneumonia and sepsis in both the treatment arm. A meta-analysis of 6 studies also have shown lack of association of beta blocker with infection, pneumonia and sepsis [30]. Three of these studies favored benefit of beta-blocker in preventing pneumonia and stroke associated infection [14,15,24], 2 studies have reported increased risk [31,32], and one did not find increased risk of infection [33]. The benefit of beta blocker in stroke has been attributed to reduced sympathetic drive, myocardial arrhythmia, oxygen demand, heart failure, sepsis and acute respiratory distress syndrome [34-37]. Increased adrenaline impairs Th1 cell differentiation but not Th2 cells resulting in imbalance between Th1 and Th2 cell population. Adrenalines also suppress bone marrow and thymus; thereby, stroke-associated infections [11]. Beta blocker in recommended dose may not be sufficient to suppress the amount of catecholamine released in stroke, more so in moderate to large strokes.
In our study, GCS score was associated with mortality, and beta blocker and SIRS at 15 days were associated with functional outcome. Multiple studies have reported GCS score, volume of stroke, NIHSS score, mid-line shift, herniation, SIRS, oxidative stress markers and catecholamine as predictors of death and disability [24,38-45]. Glasgow Coma Scale is an age old robust clinical scale for evaluation of unconscious [46], and has been a predictor of not only stroke but also for multiple causes of acute brain insult such as head injury [47], subarachnoid hemorrhage [48], cerebral venous sinus thrombosis, acute encephalitis ad meningitis [49,50]. SIRS at 15 days may suggest infection or ongoing immune-mediated delayed brain edema [51].
Limitations
This study includes both infarctions and ICH which have different pathophysiology. Moreover, the severity of stroke is also different. Smaller sample size makes sub-analysis under-powered. We did not consider reduction in blood pressure as an outcome measure. Biomarkers of stress were also not evaluated.
Highlights
- Metoprolol in acute stroke has a disability benefit at 3 months
- Death is similar between metoprolol and amlodipine group.
- Stroke associated infections are similar between the two treatment arms.
- Depth of coma predicted mortality.
- 3-month outcome was associated with use of metoprolol, and SIRS at 7th and 15th day.
Metoprolol is associated with good recovery of surviving patients at 3 months compared to amlodipine. However, they should be closely monitored for bradycardia. A large multi-centric, double-blind, randomized controlled trial is needed to validate these observations. Future studies including biomarkers of stress may help clarify the mechanisms underlying beta-blocker benefits in stroke.
We thank Mrs. Anam Siddiqui for secretarial assistance.
Data availability statement: The data supporting the results of this investigation are available from the first author upon reasonable request.
Ethical compliance statement: This study was approved by the Institutional Ethics Committee, SGPGIMS, Lucknow.
Consent statement: Informed consent was obtained from the patients or their legal guardians.
Ethical standard: The protocol was approved by the Institute Ethics Committee (Ethics No: 2022-20-IMP-125 08/07/2022) and registered in Clinical Trial Registry-India (CTRI link: 2022/07/043879).
Author contributions
Jayantee Kalita - Conceptualization, Writing and reviewing original draft.
Dhiraj Kumar - Data curation, Formal analysis
Nagendra B Gutti - Data curation, Formal analysis
Sandeep K Gupta - Data curation, Formal analysis
Anadi Mishra - Data curation, Formal analysis
Vivek Singh - Visualization.
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795-820. Available from: https://doi.org/10.1016/s1474-4422(21)00252-0
- Kalita J, Goyal G, Kumar P, Misra UK. Intracerebral hemorrhage in young patients from a tertiary neurology center in North India. J Neurol Sci. 2014;336(1-2):42-7. Available from: https://doi.org/10.1016/j.jns.2013.09.037
- Hankey GJ. Population Impact of Potentially Modifiable Risk Factors for Stroke. Stroke. 2020;51(3):719-728. Available from: https://doi.org/10.1161/strokeaha.119.024154
- Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315-53. Available from: https://doi.org/10.1161/01.str.0000437068.30550.cf
- Algin A, Inan I. The role of radiologic, clinical and biochemical parameters in prediction of stroke mortality. Neurosciences (Riyadh). 2019;24(2):110-114. Available from: https://doi.org/10.17712/nsj.2019.2.20180021
- Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ Res. 2017;121(4):451-468. Available from: https://doi.org/10.1161/circresaha.117.311170
- Maier IL, Karch A, Mikolajczyk R, Bähr M, Liman J. Effect of beta-blocker therapy on the risk of infections and death after acute stroke--a historical cohort study. PLoS One. 2015;10(2):e0116836. Available from: https://doi.org/10.1371/journal.pone.0116836
- Dorrance AM, Fink G. Effects of Stroke on the Autonomic Nervous System. Compr Physiol. 2015;5(3):1241-63. Available from: https://doi.org/10.1002/cphy.c140016
- Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178:101610. Available from: https://doi.org/10.1016/j.pneurobio.2019.03.003
- Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int J Mol Sci. 2020;21(18):6454. Available from: https://doi.org/10.3390/ijms21186454
- Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725-36. Available from: https://doi.org/10.1084/jem.20021098
- Koton S, Tanne D, Green MS, Bornstein NM. Mortality and predictors of death 1 month and 3 years after first-ever ischemic stroke: data from the first national acute stroke Israeli survey (NASIS 2004). Neuroepidemiology. 2010;34(2):90-6. Available from: https://doi.org/10.1159/000264826
- Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JR. Low dose beta blockade in acute stroke ("BEST" trial): an evaluation. Br Med J (Clin Res Ed). 1988;296(6624):737-41. Available from: https://doi.org/10.1136/bmj.296.6624.737
- Dziedzic T, Slowik A, Pera J, Szczudlik A. Beta-blockers reduce the risk of early death in ischemic stroke. J Neurol Sci. 2007;252(1):53-6. Available from: https://doi.org/10.1016/j.jns.2006.10.007
- Sykora M, Siarnik P, Diedler J; VISTA Acute Collaborators. β-Blockers, Pneumonia, and Outcome After Ischemic Stroke: Evidence From Virtual International Stroke Trials Archive. Stroke. 2015;46(5):1269-74. Available from: https://doi.org/10.1161/strokeaha.114.008260
- Balla HZ, Cao Y, Ström JO. Effect of Beta-Blockers on Stroke Outcome: A Meta-Analysis. Clin Epidemiol. 2021 Mar 16;13:225-236. Available from: https://doi.org/10.2147/clep.s268105
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117-23. Available from: https://pubmed.ncbi.nlm.nih.gov/7799491/
- Misra UK, Kalita J, Srivastava M, Mandal SK. A study of prognostic predictors of supratentorial haematomas. J Neurol. 1996;243(1):96-100. Available from: https://doi.org/10.1007/bf00878539
- de Margerie-Mellon C, Turc G, Tisserand M, Naggara O, Calvet D, Legrand L, et al. Can DWI-ASPECTS substitute for lesion volume in acute stroke? Stroke. 2013 Dec;44(12):3565-7. Available from: https://doi.org/10.1161/strokeaha.113.003047
- Mandell, Lionel A, and Michael S. Niederman. "Pneumonia." Harrison's Principles of Internal Medicine, 21e. McGraw-Hill Education, 2022. Available from: https://ugc.production.linktr.ee/d40733b1-ae5f-4b54-a04a-59f8dda7e3a8_1-Reduced-Harrison-21st.pdf
- Brant EB, Seymour CW, Angus DC. Sepsis and Septic Shock. In: Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson J, editors. Harrison's Principles of Internal Medicine, 21e. McGraw-Hill Education; 2022. Accessed March 06, 2025.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-10. Available from: https://doi.org/10.1001/jama.2016.0287
- Kalita J, Bastia J, Bhoi SK, Misra UK. Systemic Inflammatory Response Syndrome Predicts Severity of Stroke and Outcome. J Stroke Cerebrovasc Dis. 2015;24(7):1640-8. Available from: https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.03.057
- Kalita J, Misra UK, Kumar B. Is β-blocker (atenolol) a preferred antihypertensive in acute intracerebral hemorrhage? Neurol Sci. 2013;34(7):1099-104. Available from: https://doi.org/10.1007/s10072-012-1210-y
- Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al. Controlling hypertension and hypotension immediately post stroke (CHHIPS)--a randomised controlled trial. Health Technol Assess. 2009;13(9):iii, ix-xi, 1-73. Available from: https://doi.org/10.3310/hta13090
- Savitz SI, Erhardt JA, Anthony JV, Gupta G, Li X, Barone FC, et al. The novel beta-blocker, carvedilol, provides neuroprotection in transient focal stroke. J Cereb Blood Flow Metab. 2000;20(8):1197-204. Available from: https://doi.org/10.1097/00004647-200008000-00005
- Tziomalos K, Giampatzis V, Bouziana SD, Spanou M, Papadopoulou M, Kazantzidou P, et al. Effects of different classes of antihypertensive agents on the outcome of acute ischemic stroke. J Clin Hypertens (Greenwich). 2015;17(4):275-80. Available from: https://doi.org/10.1111/jch.12498
- Sundbøll J, Schmidt M, Horváth-Puhó E, Christiansen CF, Pedersen L, Bøtker HE, et al. Impact of preadmission treatment with calcium channel blockers or beta blockers on short-term mortality after stroke: a nationwide cohort study. BMC Neurol. 2015;15:24. Available from: https://doi.org/10.1186/s12883-015-0279-3
- Eizenberg Y, Grossman E, Tanne D, Koton S. Pre-admission treatment with Beta-blockers in hypertensive patients with acute stroke and 3-month outcome-Data from a national stroke registry. J Clin Hypertens (Greenwich). 2018;20(3):568-72. Available from: https://doi.org/10.1111/jch.13211
- Yang L, Wenping X, Jinfeng Z, Jiangxia P, Jingbo W, Baojun W. Are beta blockers effective in preventing stroke-associated infections? - a systematic review and meta-analysis. Aging (Albany NY). 2022;14(10):4459-70. Available from: https://doi.org/10.18632/aging.204086
- Starr JB, Tirschwell DL, Becker KJ. Increased infections with β-blocker use in ischemic stroke, a β2-receptor mediated process? Neurol Sci. 2017;38(6):967-74. Available from: https://doi.org/10.1007/s10072-017-2877-x
- Westendorp WF, Vermeij JD, Brouwer MC, Roos YB, Nederkoorn PJ, van de Beek D; PASS Investigators. Pre-Stroke Use of Beta-Blockers Does Not Lower Post-Stroke Infection Rate: An Exploratory Analysis of the Preventive Antibiotics in Stroke Study. Cerebrovasc Dis. 2016;42(5-6):506-11. Available from: https://doi.org/10.1159/000450926
- Maier IL, Becker JC, Leyhe JR, Schnieder M, Behme D, Psychogios MN, et al. Influence of beta-blocker therapy on the risk of infections and death in patients at high risk for stroke induced immunodepression. PLoS One. 2018;13(4):e0196174. Available from: https://doi.org/10.1371/journal.pone.0196174
- Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318(7200):1730-7. Available from: https://doi.org/10.1136/bmj.318.7200.1730
- Heidenreich PA, Lee TT, Massie BM. Effect of beta-blockade on mortality in patients with heart failure: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 1997;30(1):27-34. Available from: https://doi.org/10.1016/s0735-1097(97)00104-6
- Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683-91. Available from: https://doi.org/10.1001/jama.2013.278477
- van der Jagt M, Miranda DR. Beta-blockers in intensive care medicine: potential benefit in acute brain injury and acute respiratory distress syndrome. Recent Pat Cardiovasc Drug Discov. 2012;7(2):141-51. Available from: https://doi.org/10.2174/157489012801227274
- Weir CJ, Bradford AP, Lees KR. The prognostic value of the components of the Glasgow Coma Scale following acute stroke. QJM. 2003;96(1):67-74. Available from: https://doi.org/10.1093/qjmed/hcg008
- Das S, Chandra Ghosh K, Malhotra M, Yadav U, Sankar Kundu S, Kumar Gangopadhyay P. Short-term mortality predictors in acute stroke. Ann Neurosci. 2012;19(2):61-7. Available from: https://doi.org/10.5214/ans.0972.7531.12190203
- Saunders DE, Clifton AG, Brown MM. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke. 1995;26(12):2272-6. Available from: https://doi.org/10.1161/01.str.26.12.2272
- Mahdy ME, Ghonimi NA, Elserafy TS, Mahmoud W. The NIHSS score can predict the outcome of patients with primary intracerebral hemorrhage. Egypt J Neurol Psychiatry Neurosurg. 2019;55:21. Available from: https://ejnpn.springeropen.com/articles/10.1186/s41983-019-0056-0
- McKeown ME, Prasad A, Kobsa J, Top I, Snider SB, Kidwell C, et al. Midline shift greater than 3 mm independently predicts outcome after ischemic stroke. Neurocrit Care. 2022;36(1):46-51. Available from: https://doi.org/10.1007/s12028-021-01341-x
- Yuan MZ, Li F, Fang Q, Wang W, Peng JJ, Qin DY, et al. Research on the cause of death for severe stroke patients. J Clin Nurs. 2018;27(1-2):450-60. Available from: https://doi.org/10.1111/jocn.13954
- Chen YC, Chen CM, Liu JL, Chen ST, Cheng ML, Chiu DT. Oxidative markers in spontaneous intracerebral hemorrhage: leukocyte 8-hydroxy-2'-deoxyguanosine as an independent predictor of the 30-day outcome. J Neurosurg. 2011;115(6):1184-90. Available from: https://doi.org/10.3171/2011.7.jns11718
- Feibel JH, Hardy PM, Campbell RG, Goldstein MN, Joynt RJ. Prognostic value of the stress response following stroke. JAMA. 1977;238(13):1374-6. Available from: https://pubmed.ncbi.nlm.nih.gov/578192/
- Mehta R, Chinthapalli K. Glasgow coma scale explained. BMJ. 2019;365:l1296. Available from: https://doi.org/10.1136/bmj.l1296
- McNett M. A review of the predictive ability of Glasgow Coma Scale scores in head-injured patients. J Neurosci Nurs. 2007;39(2):68-75. Available from: https://doi.org/10.1097/01376517-200704000-00002
- Hanel RA, Xavier AR, Mohammad Y, Kirmani JF, Yahia AM, Qureshi AI. Outcome following intracerebral hemorrhage and subarachnoid hemorrhage. Neurol Res. 2002;24 Suppl 1:S58-62. Available from: https://doi.org/10.1179/016164102101200041
- Kalita J, Mani VE, Bhoi SK, Misra UK. Spectrum and outcome of acute infectious encephalitis/encephalopathy in an intensive care unit from India. QJM. 2017;110(3):141-8. Available from: https://doi.org/10.1093/qjmed/hcw132
- Kalita J, Singh RK, Misra UK, Kumar S. Evaluation of cerebral arterial and venous system in tuberculous meningitis. J Neuroradiol. 2018;45(2):130-5. Available from: https://doi.org/10.1016/j.neurad.2017.09.005
- Boehme AK, Hays AN, Kicielinski KP, Arora K, Kapoor N, Lyerly MJ, et al. Systemic inflammatory response syndrome and outcomes in intracerebral hemorrhage. Neurocrit Care. 2016;25(1):133-40. Available from: https://doi.org/10.1007/s12028-016-0255-9