More Information
Submitted: March 11, 2025 | Approved: March 19, 2025 | Published: March 20, 2025
How to cite this article: Abdelmissih S, Gamal1 M, Naeem KM. Pyridostigmine-Induced Status Epilepticus Rat Model Was Resistant to Increasing Doses of Ramipril: The Latter Triggered Epileptogenesis, Arrhythmia, and Cardiac Ischemia in a Dose-Dependent Manner. J Neurosci Neurol Disord. 2025; 9(1): 010-027. Available from:
https://dx.doi.org/10.29328/journal.jnnd.1001106
DOI: 10.29328/journal.jnnd.1001106
Copyright License: © 2025 Abdelmissih S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ramipril; Pyridostigmine; Sodium valproate; Racine’ scoring; Status epilepticus; Epileptogenesis; EEG; ECG; Ischemia; Arrhythmia
Pyridostigmine-Induced Status Epilepticus Rat Model Was Resistant to Increasing Doses of Ramipril: The Latter Triggered Epileptogenesis, Arrhythmia, and Cardiac Ischemia in a Dose-Dependent Manner
Sherine Abdelmissih*, Monica Gamal and Kerollos M Naeem
Department of Medical Pharmacology, Faculty of Medicine, Kasr Al-Ainy, Cairo University, Cairo, Egypt
*Address for Correspondence: Sherine Abdelmissih, Department of Medical Pharmacology, Faculty of Medicine, Kasr Al-Ainy, Cairo University, Cairo, Egypt, Email: [email protected]; [email protected]
Background: Studies explored the therapeutic role of agents inhibiting RAS in epilepsy. Fewer studies addressed the electrophysiological changes associated with angiotensin converting enzyme inhibitors (ACEIs) in terms of sustained seizures (status epilepticus). Sodium valproate (SVPA), a broad-spectrum anticonvulsant, has been associated with adverse cardiac events upon long-term use, in contrast to the beneficial role of ACEIs in cardiovascular disorders. This work explored the potential effects of ramipril, an ACEI, compared to SVPA, on the behavior, and electrophysiology of the brain and heart in a rat model of status epilepticus. The dose dependent pattern of the presumed ramipril activities was investigated.
Methods: Adult male rats were assigned into seven groups, controls, IP pyridostigmine (36 mg/kg)-induced status epilepticus (PISE), oral SVPA (5 mg/kg), and three groups receiving oral ramipril at respective doses of 5 (R5), 10 (R10), and 20 mg/kg (R20). Rat behavior was assessed using Racine’s motor convulsion scoring for 10 minutes. Blood pressure was recorded, and electroencephalography (EEG) and electrocardiography (ECG) were performed on the sedated rats 24 hours after recovery.
Results: Despite the partial behavioral improvement of motor convulsions with R5 and R10 exhibited epileptogenic activity, as indicated by the increased relative power of fast and slow gamma waves and total EEG power. R10 triggered arrhythmia and cardiac ischemia as indicated by absence of P wave, along with ST elevation and tall T wave, slowed heart rate and prolonged QRS, QTc, and RR intervals.
Conclusion: PISE was resistant to sodium valproate and ramipril. Ramipril at low and moderate doses induced epileptogenic activity and, especially at moderate dose, precipitated cardiac ischemia and arrhythmia.
Summary
The debatable role of ramipril in epilepsy was studied in a rat model of pyridostigmine-induced status epilepticus, compared to sodium valproate. Increasing ramipril doses did not resolve status epilepticus in rats. Instead, low and moderate doses exhibited epileptogenic activity, opposite to high dose ramipril and sodium valproate. Blood pressure was dose-dependently reduced with ramipril. Electrocardiography showed evidence of cardiac arrythmia and ischemia, especially with the moderate ramipril dose. The behavioral and EEG indices correlated with systolic blood pressure and ECG changes.
Epilepsy is one of the most common global neurological disorders, affecting around 50 million people worldwide. Epilepsy adversely affects the productive life of humans by disturbing the normal motor, cognitive, and emotional development, along with being associated with an increased risk of mortality, reaching up to three times higher incidence than the general population [1]. Epileptogenesis is the functional abnormality of neuronal networks increasing seizure susceptibility [2] which precedes the symptomatic reveal of epilepsy and manifests as abnormal electroencephalography (EEG) changes. Addressing epileptogenesis seems crucial to seizure control, being identified as a continuous, progressive process that extends beyond the occurrence of seizures. This identification makes detecting epileptogenesis an appealing aspect of pharmacologic epilepsy control [3]. EEG can detect epileptiform activity even in absence of seizures, offering early detection and management of epilepsy in asymptomatic cases or the resumption of AEDs after withdrawal in seizure-free patients [4]. Scalp EEG allows recordings on a large scale, reflecting summative electrical discharges from indefinite neuronal networks [5]. Nonetheless, therapeutic responses should not be judged, solely, upon EEG due to the contribution of other risk factors, including, among others, genetic factors [6]; hence, our study adopted Racine’s scoring system for behavioral assessment of motor convulsions.
Despite the availability of multiple antiepileptic medications, resistant cases persist , yielding the so called ‘intractable’ or ‘pharmaco-resistant’ epilepsy [7], leading to the potentially lethal status epilepticus (SE). SE is defined as either a convulsive or a non-convulsive episode lasting for ≥ 5 minutes, or recurrent seizure activity without regaining conscious level in between (recovery) [8]. The notion that some antiepileptic drugs (AEDs) can target the renin-angiotensin system (RAS) [9,10] has driven the attention towards the exploration of this system in terms of epileptogenesis and epilepsy. Some studies highlighted the potential ability of phenobarbital, benzodiazepines, and acetazolamide-all of which are known AEDs- to alter the RAS [11,12]. In this context, serum renin was elevated in patients having seizures while serum level of phenobarbital was low [13]. In rat’s brain, the inhibitory activity of the AEDs, diazepam and carbamazepine, over the angiotensin converting enzyme (ACE)- the enzyme responsible for angiotensin II (Ang II) synthesis- [14-16], and, conversely, the favorable effects of RAS inhibitors in curing epilepsy [9,17,18] underpinned the possible involvement of RAS in epileptogenesis. Despite that Ang II was regarded as a proconvulsant factor which inhibition by angiotensin converting enzyme inhibitors (ACEIs) yielded favorable outcomes in epilepsy control, ACEIs of the sulfhydryl group, such as captopril and zofenopril, and those of the non-sulfhydryl group, such as enalapril and fosinopril, did not have significant anticonvulsant activity in a mouse model of audiogenic-stimulated seizures [19]. Even that ACEIs can precipitate seizures [20] as rationalized by a peripheral-to-central escape of angiotensin I, thus enhancing the central conversion to Ang II. Nonetheless, the antiepileptic activity of one of the broad-spectrum AEDs, valproic acid, was potentiated by RAS inhibitors [21,22]. Thus, comparing the valproic acid derivative, sodium valproate (SVPA), to ramipril, one of the less studied ACEIs in terms of epilepsy, seemed appealing.
Furthermore, the link between some cardiovascular disorders, including ischemic heart diseases, and arrhythmia, and epilepsy [23-25] has guided our study to investigate the link between the cardiovascular effects of ramipril to its presumed activity in epilepsy. More importantly, seizures seemed a threat to the heart and can trigger cardiomyocyte injury as well as arterial hypertension [26,27]. Some of the epilepsy-triggered arrhythmia involved atrial fibrillation, bradyarrhythmia and ventricular arrhythmia [28-30]. These cardiac complications are potential contributors to sudden unexpected death, with an incidence of 0.09 to 2.4 per 1000 person-years [31,32]. The need to compare the antiepileptic activity of ramipril to SVPA in terms of cardiovascular events stems from the reported increased risk of long-term arrhythmia in SVPA-treated patients with epilepsy and a three-fold increase in ventricular fibrillation-related sudden cardiac death [33]. Still, studies are lacking as regards the acute cardiovascular effects of SVPA in the peri-ictal period of status epilepticus.
Therefore, we compared the effects of ramipril to SVPA in a pyridostigmine-induced status epilepticus (PISE) in adult male rats. Three different doses of ramipril were employed to assess whether presumed effects followed a dose-dependent pattern, the lowest dose is the one commonly adopted in research. Secondly, we explored whether the behavioral, EEG, and ECG changes were linked.
All experimental procedures comply with the ARRIVE guidelines and were carried out in accordance with JoVE’s animal use guidelines Animals. Animals’ housing, handling and experimentation were approved by the Institutional Animal Care and Use Committee of Cairo University (CU/III/F/46/24).
Experimental design and treatments
Adult male Wistar albino rats aged 7–8 months (200–250 g) were obtained from Animal House of Faculty of Medicine, Kasr Al-Ainy, Cairo University. Animals were maintained 4-5 rats/cage in the Acclimatization Room at the Medical Pharmacology Department, Faculty of Medicine. Kasr Al-Ainy, Cairo University for 7 days before the start of the experiment, under standard conditions (22 ± 2 ℃ room temperature; 45% – 50% relative humidity; 12-hour light/dark cycle with lights on at 07:30), with access to food and water ad libitum.
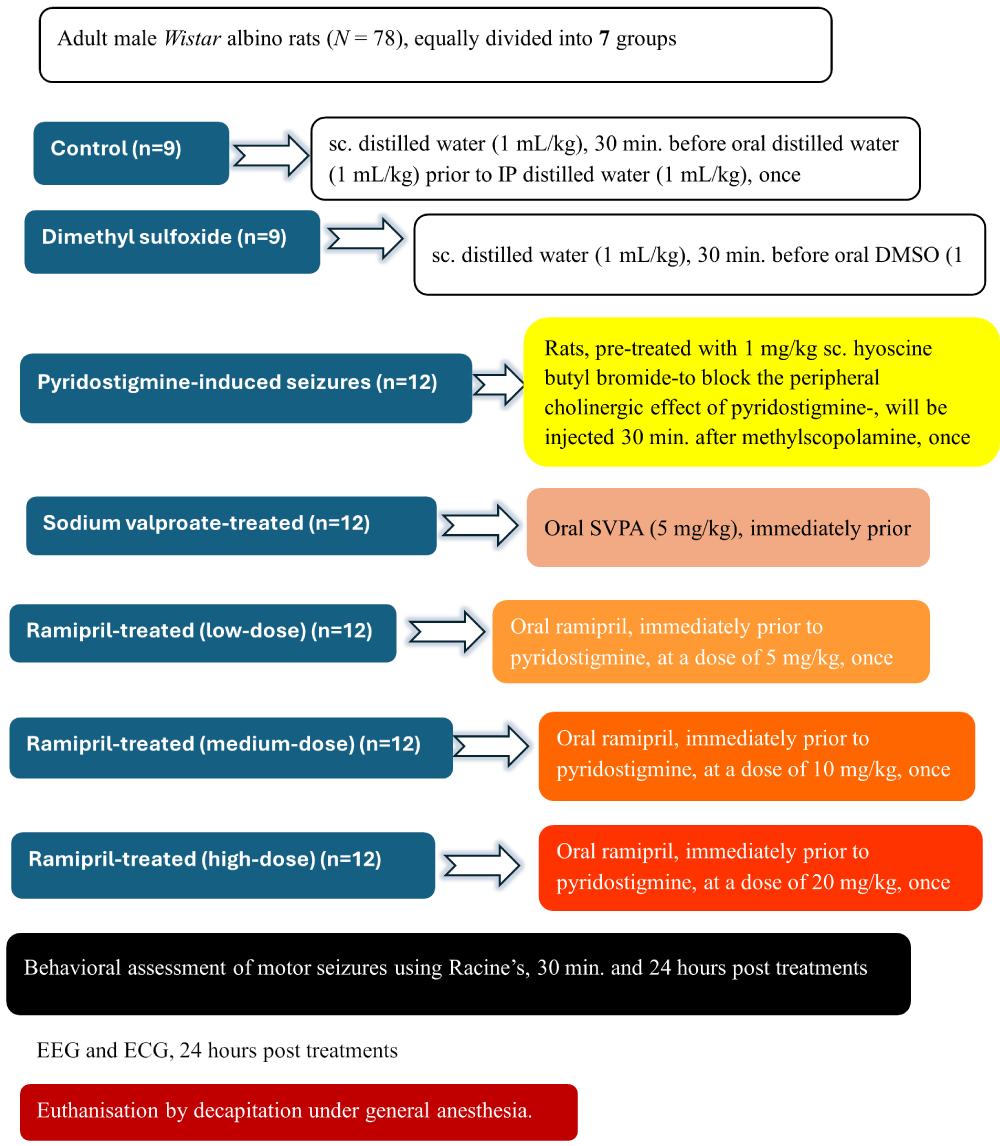
Adult male Wistar albino rats (N = 78) (250-300 g) were divided into the following seven groups: Controls (n = 9), wherein rats received sc. distilled water (1 mL/kg), 30 min. before oral distilled water (1 mL/kg), followed by IP distilled water (1 mL/kg); Dimethyl sulfoxide (DMSO) (Amazon, USA) (n = 9), wherein rats received sc. distilled water (1 mL/kg), 30 min. before oral DMSO (1 mL/kg), followed by IP distilled water (1 mL/kg); Pyridostigmine-induced seizures (PISE) (n = 12), wherein rats, pre-treated with 1 mg/kg sc. Scopolamine methyl bromide (Sigma-Aldrich Co., St. Louis, USA) -to block the peripheral cholinergic effect of pyridostigmine- [34], were injected, 30 min. before IP pyridostigmine bromide (Sigma-Aldrich Co., St. Louis, USA) (0.5 mg/kg), guided by a pilot study starting with a dose of 36 mg/kg-based on a the relative acetylcholinesterase inhibitory activity of pyridostigmine compared to physostigmine [35]; SVPA (Sigma-Aldrich Co., St. Louis, USA) (n = 12), wherein rats were administered oral SVPA (5 mg/kg) [36], immediately prior to pyridostigmine + scopolamine methyl bromide; Ramipril (Sigma-Aldrich Co., St. Louis, USA) (n = 36), wherein rats received oral ramipril, immediately prior to pyridostigmine + scopolamine methyl bromide, at respective doses of 5mg/kg designated as R5, 10 mg/kg designated as R10, and 20 mg/kg, designated as R20 [37]. All treatments were received once, after 12-hour fasting as food delays the oral absorption of SVPA [38]. No pharmacokinetic interactions were observed between pyridostigmine and either SVPA or ramipril. As a rescue medication in status epilepticus, IP lorazepam (Pfizer, USA) (0.94 mg/kg) was available [39]. In case of cardiac arrest, IM atropine sulfate (Sigma-Aldrich Co., St. Louis, USA) (0.5 mg/kg) was available [40] (Figure 1).
Figure 1: Experimental design and animal grouping. Adult male Wistar albino rats (N = 78) were divided into seven groups. DMSO: Dimethyl-sulfoxide; SVPA: Sodium Valproate; EEG: Electroencephalography; ECG: Electrocardiography.
Racine’s scoring for the behavioral assessment of motor seizures
Each rat was placed in a square glass box, measuring 30 cm × 30 cm x 30 cm (length x width x height), unseen from the other counter peers of the same cage, to be left for 30 min. to accommodate. Starting 5 min. after treatments’ administration, the rat’s motor behavior was video recorded for 10 min. Scoring was done on scale from 0-7, where: 0 = no response; 1 = gustatory movements and scratching; 2 = tremor and hindlimb extension; 3 = head nodding and stepping backwards; 4 = rearing and forelimb clonus; 5 = rearing, clonus, and falling; 6 = whole-body jerks; and 7 = tonic-clonic seizures and ‘wet dog’ shaking [41]. After 4.59 min. of recording, in case of spontaneous termination of seizures, the rat was left for recovery for another 30 min. In case of persistent seizures lasting for ≥ 5 min., termination of seizures was done using lorazepam. The glass box was cleared with ethanol to avoid any olfactory cues.
Non-invasive blood pressure measurement
Following a 24-hour recovery period from seizures, systolic and diastolic blood pressure were recorded using rat tail plethysmography (PanLab, Spain).Rats were sedated with IP 350 mg/kg chloral hydrate (350 mg/kg). Pulse pressure was calculated as:
Pulse pressure (mmHg) = Systolic BP (mmHg) - diastolic BP (mmHg)
Electroencephalography (EEG)
Following blood pressure recording, EEG was performed during the daytime. EEG recording lasted 5 min. per rat while sedated with closed eyes using the data acquisition system PowerLab 4/30 (ML866, ADInstruments, Australia); Animal BioAmp (ML136, ADInstruments, Australia) and Lab Chart software v8.2. Subdermal scalp needle electrodes (MLA1203; ADInstruments, Australia) were inserted according to the manufacturer’s brochure (ADInstruments, Australia). Prior to recording, the setup of the instrument was adjusted as mentioned previously in [42]. Using Data Pad commands, offline analysis for the following was done: Total EEG power (V2), mean power frequency (Hz), and mean EEG amplitude (μV), of source, fast gamma, slow gamma, beta, alpha, theta, and delta waves. The relative power of each frequency band was calculated:
Relative power of band frequency = Total power of the band frequency (V2) ÷ Total power of source EEG wave(V2)
To compare the mean amplitudes of EEG waves among the studied groups, the absolute numerical values were adopted in statistical analysis. The needle electrodes were swabbed with alcohol pad in between each rat.
Electrocardiography (ECG)
Following EEG recordings, ECG was performed using the data acquisition system PowerLab 4/30 (ML866, ADInstruments, Australia) during the daytime, for 5 min per rat. The software settings were adjusted as mentioned in [42] and according to the manufacturer’s brochure (ADInstruments, Australia). Channel 1 was used for recording. Using Data Pad commands, offline analysis to obtain data for heart rate (HR) (beats per minute (bpm)), amplitudes (millivolts) of P, Q, R, S, ST, and T waves, and duration (seconds) of P wave, PR interval, QRS complex, QT, QTc, JT, Tpeak-Tend, and RR intervals, and calculated JTc. (P wave = Atrial contraction; PR interval = Atrio-ventricular conduction; Q wave = The negative deflection that precedes the R wave; R wave = Positive upward deflection that follows the Q wave; S wave = Small negative wave following the R wave; QRS = Duration of ventricular contraction; T wave = Ventricular relaxation; QT = Time from start of ventricular contraction until the end of ventricular relaxation; QTc = Time from start of ventricular contraction until the end of ventricular relaxation (corrected for heart rate); J wave = Point at the end of QRS; JT interval = Ventricular repolarization, from end of ventricular contraction (QRS) until the end of ventricular relaxation; JTc = Ventricular repolarization, from end of ventricular contraction (QRS) until the end of ventricular relaxation (corrected for heart rate); ST segment = The interval between ventricular depolarization and ventricular repolarization (line between J point and start of T wave); Tpeak-Tend = Transmural dispersion of ventricular repolarization. The Averaging view was preset at rat ECG values and set to average over 1 second. The needle electrodes were swabbed with alcohol pad in between each rat. After completion of all experimental procedures, rats were euthanized by decapitation under general anesthesia using IP ketamine/xylazine (40/5 mg/kg) [43].
JTc (seconds) = QTc (seconds) - QRS duration (seconds)
Statistical analysis
Sample size calculation was done using G*Power software v. 3.1.9.4., at level of significance alpha = 0.05 and power (1-beta) = 0.80. Sample size was increased adopting the mortality rate encountered in the PISE model during pilot study. Data were coded and entered using GraphPad Prism v. 10.4.0 (IBM Corp., Armonk, NY, USA). The distribution of data was assessed using the Kolmogorov-Smirnov and the Sapiro-Wilk tests. Normally- distributed quantitative variables were presented as mean ± standard deviation (SD), while non- normally distributed quantitative variables were presented as median and interquartile range. Categorical variables were presented as relative frequencies. Pairwise comparisons were done using analysis of variance (ANOVA) with multiple comparisons post hoc Tukey’s test for the normally distributed quantitative variables; Brown-Forsythe and Welch ANOVA for the normally distributed quantitative variables with unequal variances; Kruskal-Wallis test with multiple comparisons post hoc Dunn’s test for the non- normally distributed quantitative variables. Fischer’s exact test was used for categorical variables when the expected frequency was less than 5. A P value < 0.05 was considered statistically significant. Correlations between quantitative variables were done using Spearman rho correlation coefficient. Correlation coefficient ‘r’ > 6 was considered a strong correlation.
This work was conducted to explore the effect of ramipril compared to SVPA in a rat model of PISE, pre-treated with scopolamine methyl bromide, to block the peripheral muscarinic effects of pyridostigmine. The behavioral assessment of motor seizures was done using Racine’s scoring. EEG was done as the gold standard diagnostic tool for epileptogenesis, even in absence of seizures. ECG was adopted presuming some links between cardiac and neurologic alterations in the context of seizures precipitation and mitigation, if any.
R5 reduced Racine’s score
Compared to controls, all PISE rats experienced seizures lasting more than 5 minutes (100% with a score of 7 vs. 0%, P < 0.0001). Compared to PISE, only R5, but not R10 or R20, showed a significantly lower number of rats experiencing SE, and with reduced scoring of ˂ 6 (22.22%, p < 0.01).
The success of our model was evident when finding that all rats in the PISE group exhibited generalized (score 6-7) sustained (> 5min.) seizures. Neither SVPA, R10, nor R20 mitigated PISE. R5 reduced seizure severity but remained significantly higher than in controls. Seizures were one of the reported adverse drug reactions of pyridostigmine but with less incidence compared to donepezil and rivastigmine -anti-Alzheimer’s medications of the same category-reversible anticholinesterases- [44]. Some reports indicated that toxicity from carbamate anticholinesterases is most commonly attributed to pyridostigmine, rather than other members of the same group, including physostigmine, neostigmine and echothiophate [45].
Despite that SVPA was effective in several seizure models [46,47], it was not able to prevent status epilepticus [48] as was in the PISE model. Similarly, the lack of efficacy of anticonvulsants was previously reported in male mice receiving IP pyridostigmine bromide (5 mg/kg) [49].
R5, but neither R10 nor R20, partially reduced the severity of PISE. Interestingly, angiotensin II was implicated in potentiating the inhibitory effect of GABA agonists in pentylenetetrazole-induced seizures, while increased the seizure threshold in bicuculline-induced seizures [50], perhaps rationalizing the lack of efficacy in PISE model. In contrast, IP captopril (50 mg/kg), another ACEI, exerted an antiseizure activity a kainic acid rat model as was detected behaviorally and by EEG [51,52]. Nonetheless, captopril itself, which was effective in the strychnine-induced seizure model [50] aggravated the bicuculline-induced seizures [53].
Ramipril reduced systolic and diastolic BP in a dose dependent manner
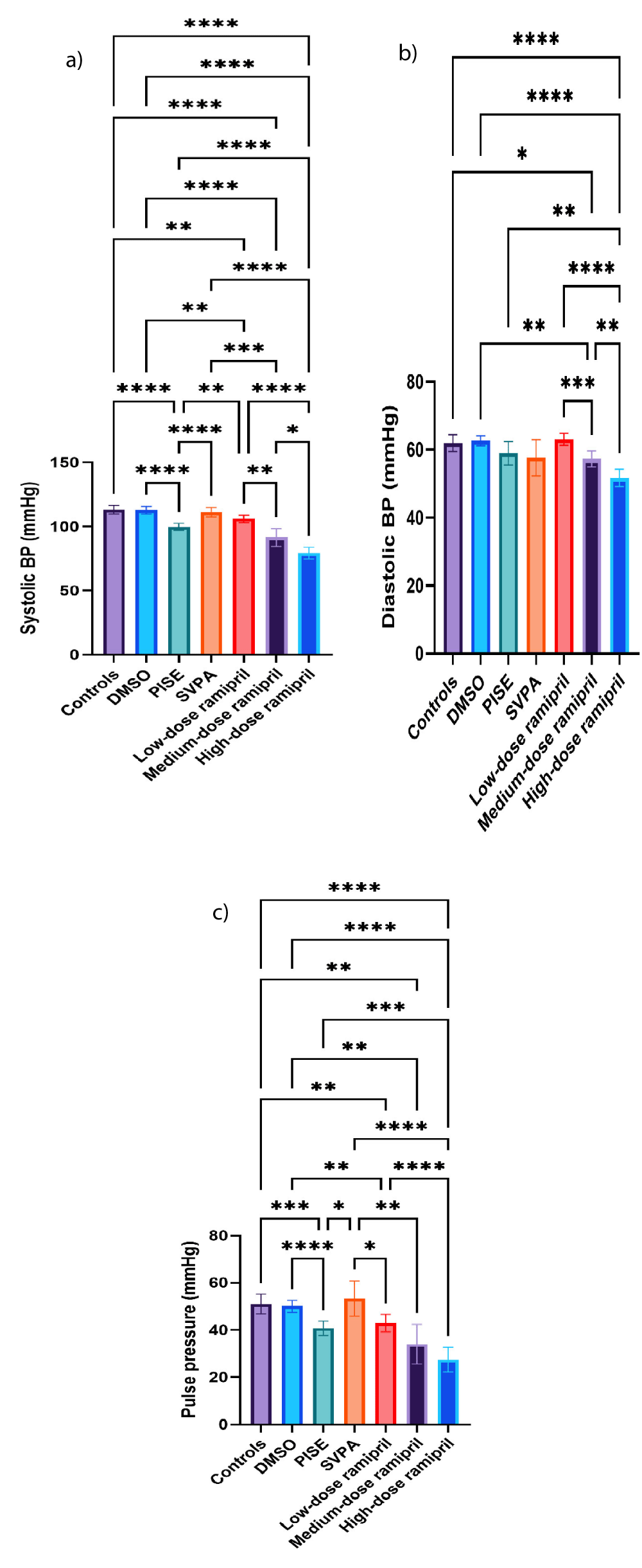
Compared to controls, PISE exhibited reduced systolic BP and pulse pressure. The reductions of systolic BP and pulse pressure with PISE were consistent with some cases of ictal hypotension, less common than ictal hypertension though. Despite blocking the peripheral anticholinergic effects using hyoscine, rats blood pressure was reduced perhaps owing to the seizure-associated fear and subsequent vasodilatation similar to vasovagal syncope [54].
On the contrary, SVPA significantly increased systolic BP and pulse pressure relative to PISE, but not to controls. Compared to controls, R5, R10, and R20 significantly reduced systolic and diastolic BP as well as pulse pressure in a dose dependent manner, as anticipated (Figures 2a-c).
Figure 2: Non-invasive blood pressure of adult male Wistar albino rats (N = 63). a. Systolic blood pressure (mmHg). b. Diastolic blood pressure (mmHg). c. Pulse pressure (mmHg). Data are expressed as mean ± SD. DMSO: dimethyl-sulfoxide; PISE: pyridostigmine-induced status epilepticus; SVPA: sodium valproate; BP: blood pressure. P ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
R5 and R10 increased the total power of source, fast gamma, and slow gamma EEG waves
Despite the overt SE exhibited in Racine’s scoring, no significant EEG changes were detected PISE following 24-hour recovery. Despite being ineffective in PISE model, SVPA reduced the total power of source wave and that of the fast gamma wave, reflecting reduced cortical excitability. Similar to the current study, SVPA did not significantly alter the power of beta and alpha waves [55]. Unlike our PISE rat model, scalp EEG recording in awake patients with eyes closed receiving SVPA showed increased power of each of theta (3.5-7.5 Hz) and delta (1.5-3.5 Hz) waves compared to untreated patients with idiopathic generalized seizures. Although not the focus of this study, fast EEG waves were linked to enhanced cognition [56], while the opposite could reflect reduced cognition which is consistent to some reports highlighting the link between valproate and cognitive dysfunction, as was detected in males with uncontrolled seizures in the dose range used herein [57].
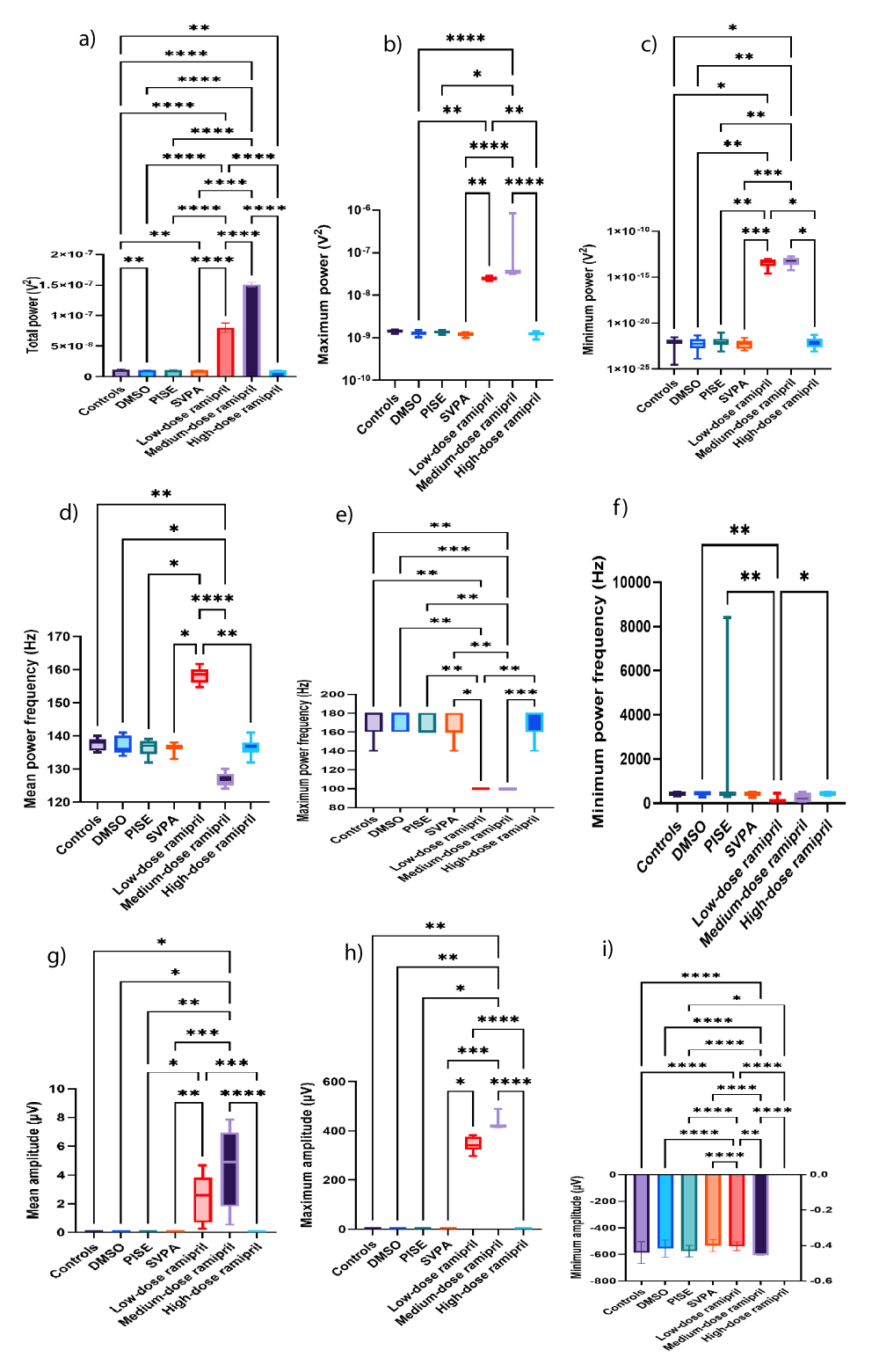
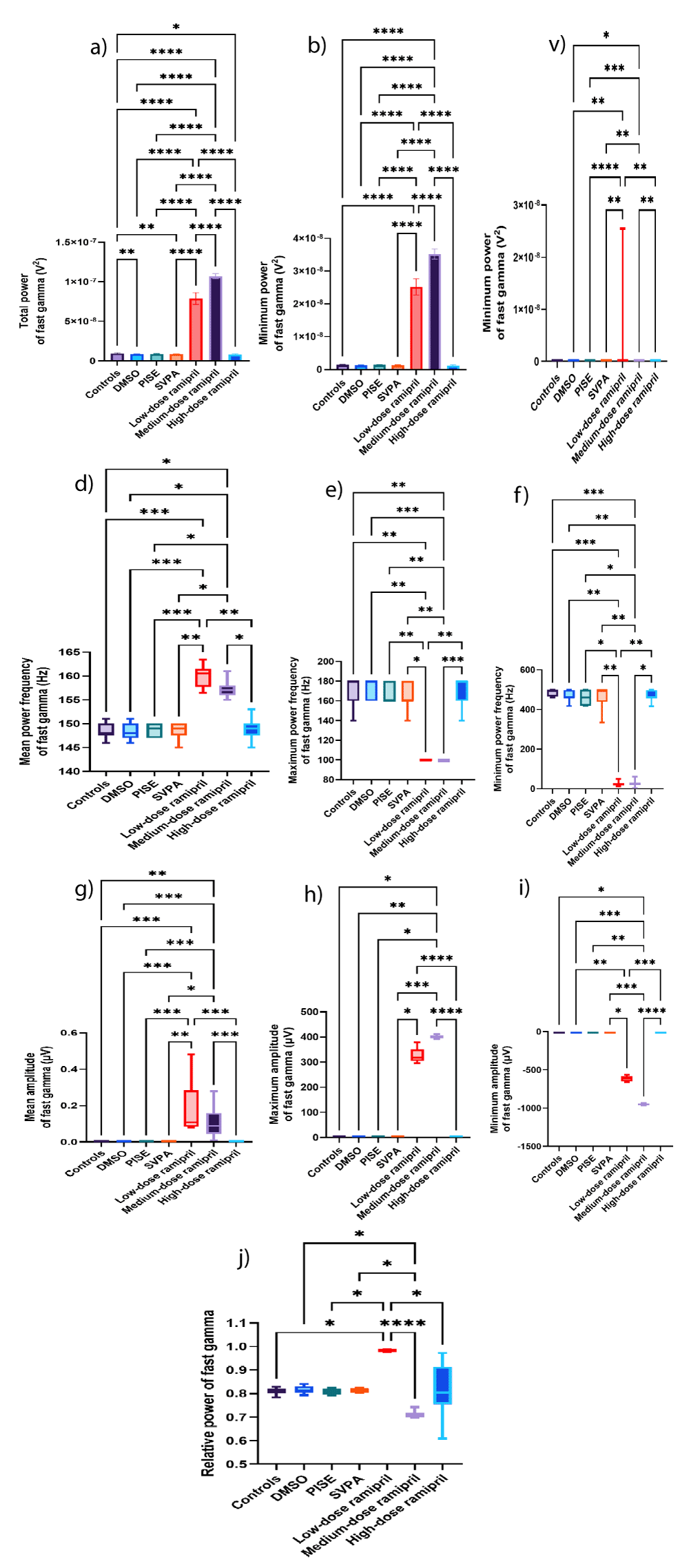
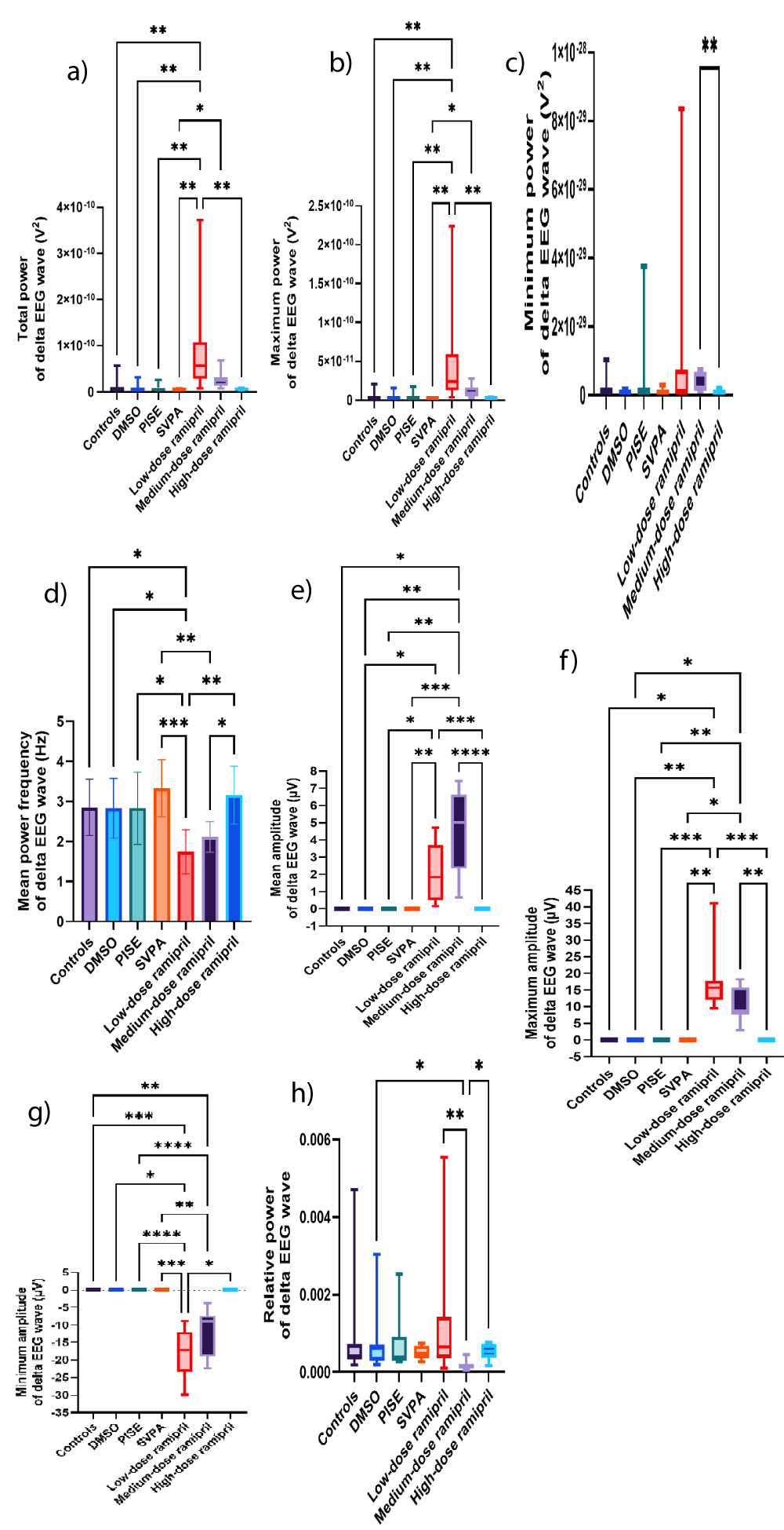
Both R5 and R10 exhibited extensive EEG changes, also opposing R20. R20 opposed R5 and R10 in terms of reducing the total power of source wave and fast gamma, as was with SVPA. Additionally, R20, but neither R5 nor R10, increased the relative power of alpha. The ability of R5 and R10, more than R20, to exert substantial EEG changes could reflect the saturation of brain transport mechanisms when increasing ramipril doses [58], or otherwise, the dose dependent reduction in BP reduced the central delivery of ramipril [59]. The increased relative power of fast and slow gamma EEG waves associated with R5 could highlight an epileptogenic activity [60-62]. This was corroborated by R5 exceeding R10 in increasing the total power of source EEG wave and the mean power frequency of fast gamma EEG wave to > 100 Hz. Also, R5 increased the total power of delta EEG wave, consistent with intracranial epileptiform discharges recorded using scalp EEG, especially at a frequency ≥ 1.4 Hz [63] similar to this work. Such an EEG slowing was reported in patients with myoclonic or non-convulsive status epilepticus as well [64] and during the post-ictal recovery period [65]. R5 exceeded R10 in increasing the amplitude of beta wave, the minimum amplitude of alpha wave and theta waves, and the maximum amplitude of theta wave. Although not the focus of this work, the increased beta amplitudes could reflect a possible anxiogenic activity attributed to ramipril [66], or it might reflect a state of enhanced cognition [67] as could be deduced from the concomitantly increased alpha and theta amplitudes [68,69], despite ongoing controversies [70,71]. R10 reduced the mean power frequency of source wave against increasing its amplitude, still in the fast, low amplitude wave range of gamma. Also, R10 increased the amplitude of fast gamma wave, added to increasing the maximum power of slow gamma wave. These changes might reflect an epileptogenic activity corroborated by the simultaneously increased delta amplitude [63]. In contrast, R10 reduced the relative powers of beta and theta waves, possibly indicating some memory impairment [72,73], although not within the scope of this research. The opposed EEG changes seen with R20 to those of R5 in terms of reduced the total power of source and fast gamma waves against increasing the relative power of alpha wave might be consistent to the lack of behavioral efficacy of R20 [74-76]. Changes in source wave, fast gamma, slow gamma, beta, alpha, theta, and delta waves are illustrated in Figures 3a-i, 4a-j, 5a-i, 6a-d, 7a-g, 8a-f, and 9a-h, respectively. Figure 10 illustrates samples of EEG recordings of the studied groups.
Figure 3: Source EEG wave of adult male Wistar albino rats (N = 63). a. Total power (V2). Data are expressed as mean ± SD. b. Maximum power (V2). c. Minimum power (V2). d. Mean power frequency (Hz). e. Maximum power frequency (Hz). f. Minimum power frequency (Hz). g. Average amplitude (µV). h. Maximum amplitude (µV). Data are expressed as median and interquartile range. i. Minimum amplitude (µV). Data are expressed as mean ± SD. DMSO: dimethyl-sulfoxide; PISE: pyridostigmine-induced status epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
Figure 4: Fast gamma EEG wave of adult male Wistar albino rats (N = 63). a. a. Total power (V2). b. Maximum power (V2). c. Minimum power (V2). Data are expressed as mean ± SD. d. Mean power frequency (Hz). e. Maximum power frequency (Hz). f. Average amplitude (µV). g. Maximum amplitude (µV). h. Minimum amplitude (µV). i. Relative power. Data are expressed as median and interquartile range. DMSO: dimethyl-sulfoxide; PISE: pyridostigmine-induced status epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
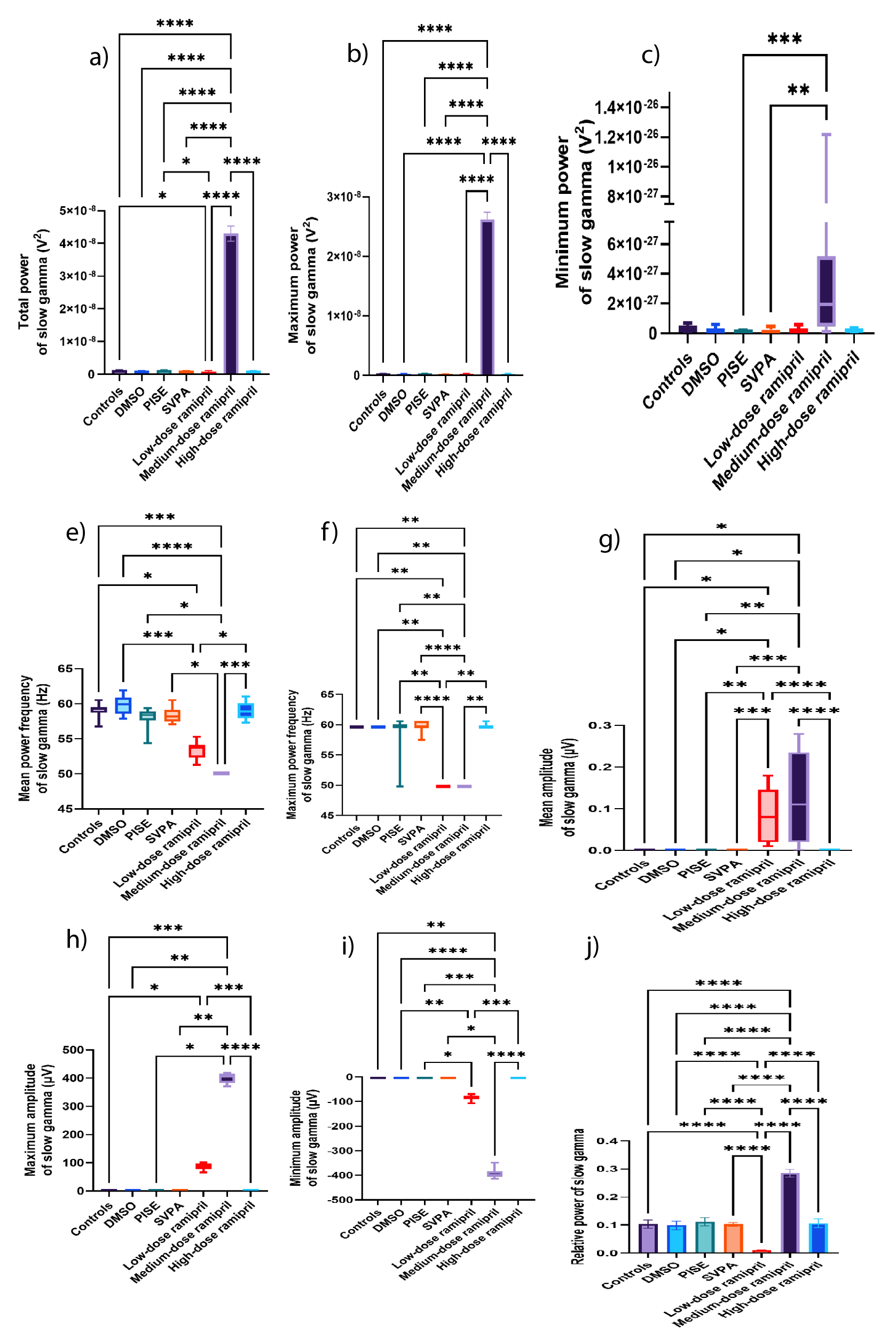
Figure 5: Slow gamma EEG wave of adult male Wistar albino rats (N = 63). a. Total power (V2). b. Maximum power (V2). Data are expressed as mean ± SD. c. Minimum power (V2). d. Mean power frequency (Hz). e. Maximum power frequency (Hz). f. Average amplitude (µV). g. Maximum amplitude (µV). h. Minimum amplitude (µV). Data are expressed as median and interquartile range. i. Relative power. Data are expressed as mean ± SD. DMSO: Dimethyl-sulfoxide; PISE: Pyridostigmine-Induced Status Epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
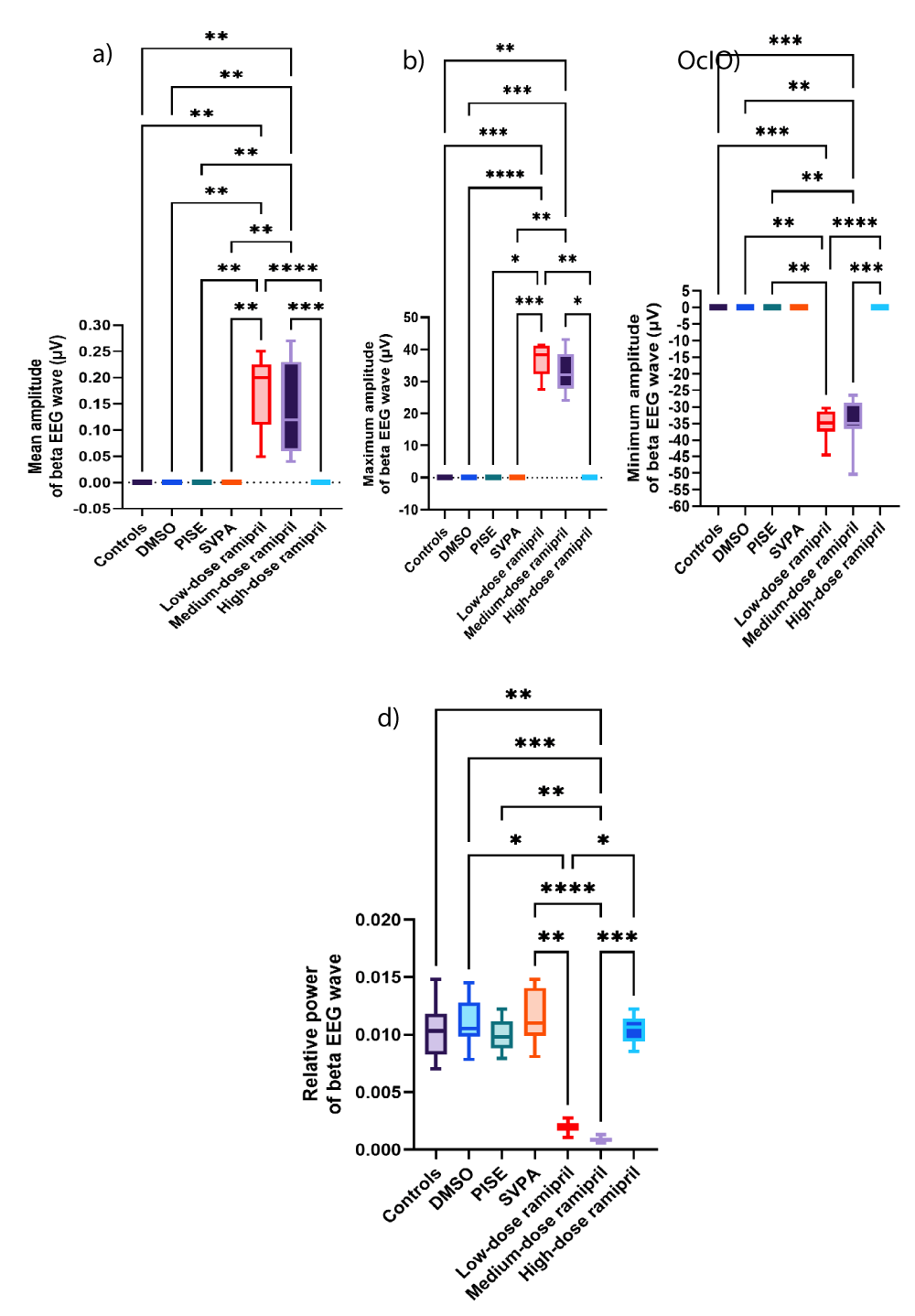
Figure 6: Beta EEG wave of adult male Wistar albino rats (N = 63). a. Average amplitude (µV). b. Maximum amplitude (µV). c. Minimum amplitude (µV). d. Relative power. Data are expressed as median and interquartile range. DMSO: dimethyl-sulfoxide; PISE: Pyridostigmine-induced Status Epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
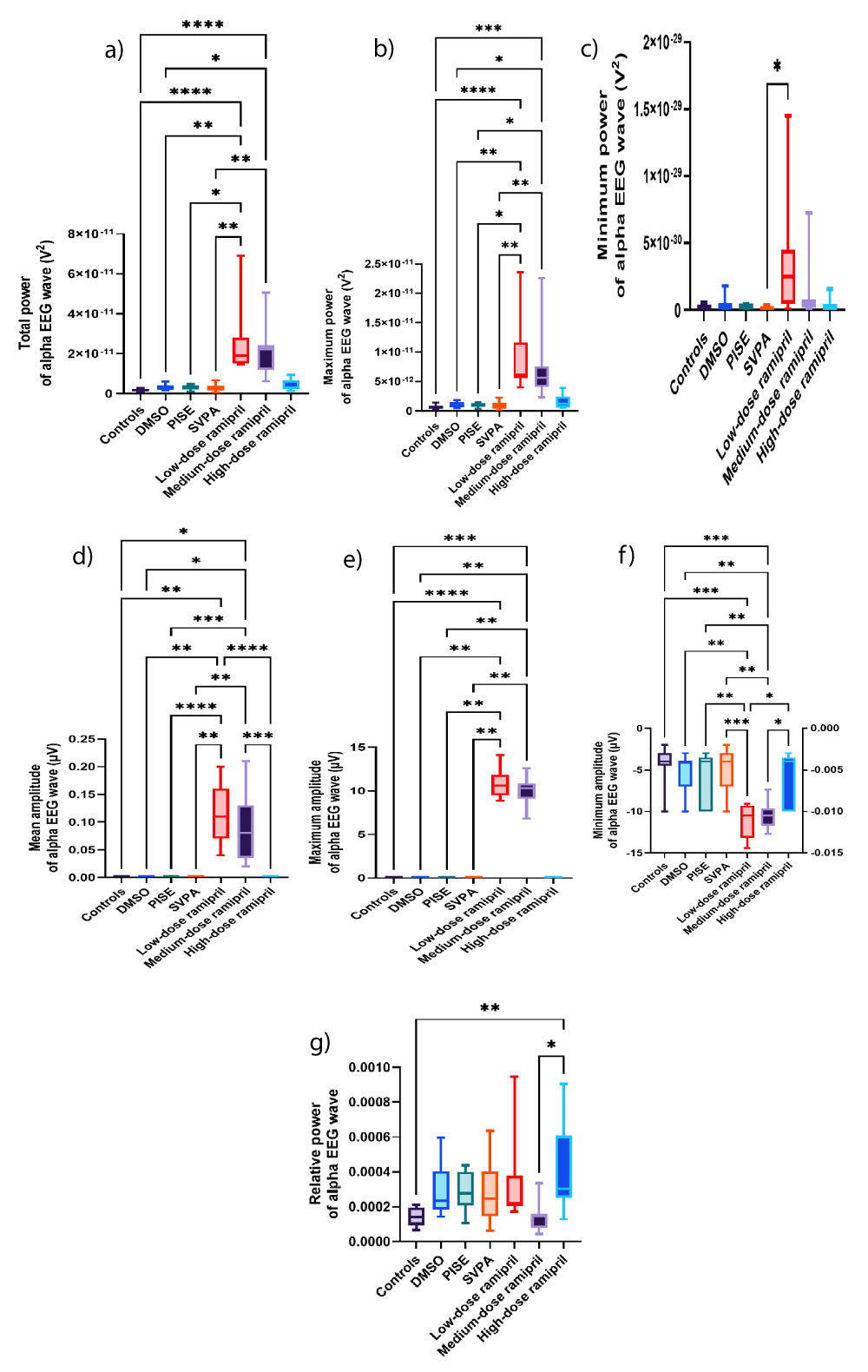
Figure 7: Alpha EEG wave of adult male Wistar albino rats (N = 63). a. Total power (V2). b. Maximum power (V2). c. Minimum power (V2). d. Average amplitude (µV). e. Maximum amplitude (µV). f. Minimum amplitude (µV). g. Relative power. Data are expressed as median and interquartile range. DMSO: Dimethyl-sulfoxide; PISE: Pyridostigmine-induced Status Epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. P ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
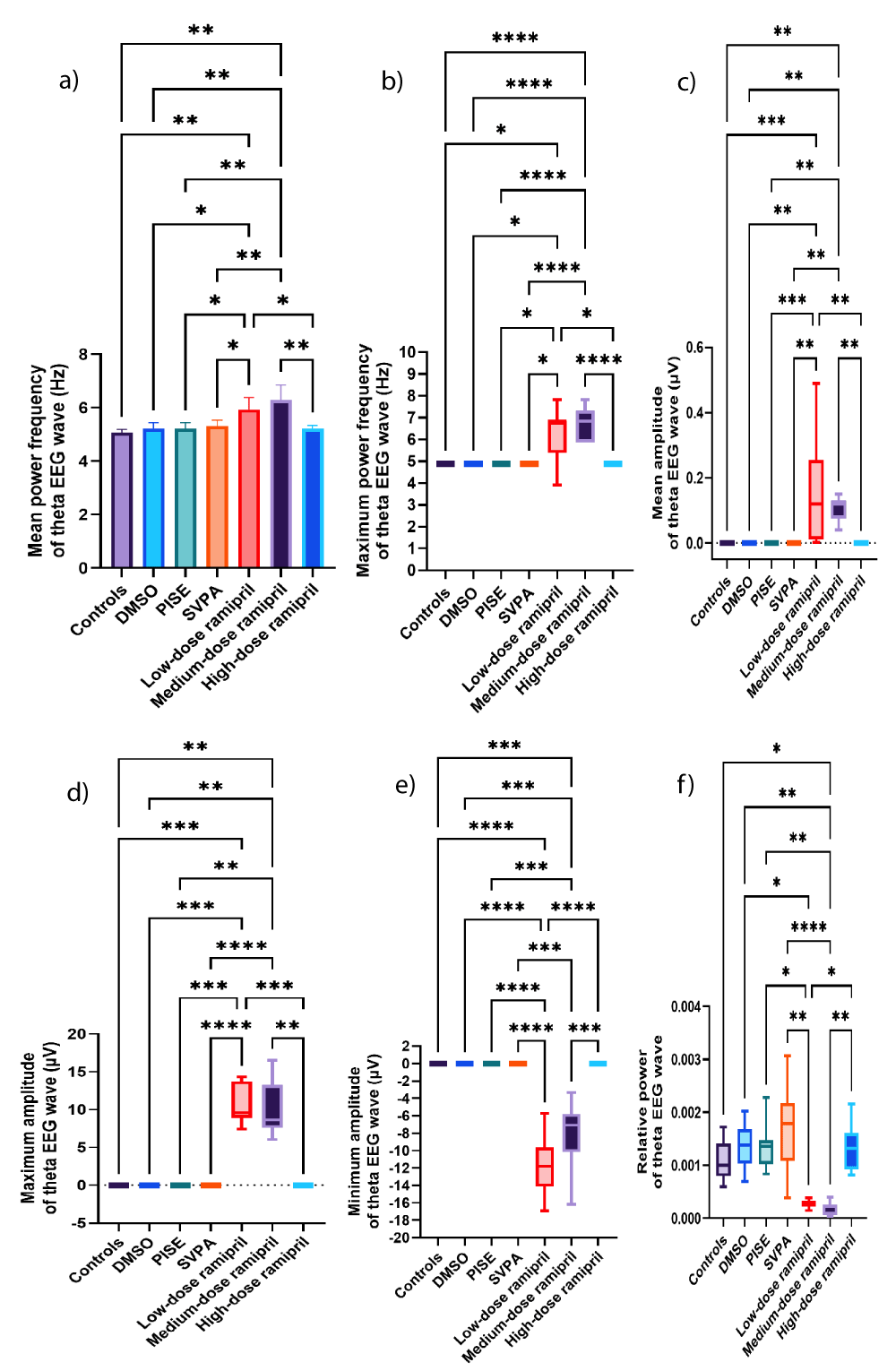
Figure 8: Theta EEG wave of adult male Wistar albino rats (N = 63). a. Mean power frequency (Hz). Data are expressed as mean ± SD. b. Maximum power frequency (Hz). c. Average amplitude (µV). d. Maximum amplitude (µV). e. Minimum amplitude (µV). f. Relative power. Data are expressed as median and interquartile range. DMSO: Dimethyl-sulfoxide; PISE: Pyridostigmine-induced Status Epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
Figure 9: Delta EEG wave of adult male Wistar albino rats (N = 63). a. Total power (V2). b. Maximum power (V2). c. Minimum power (V2). Data are expressed as median and interquartile range. d. Mean power frequency (Hz). Data are expressed as mean ± SD. e. Average amplitude (µV). f. Maximum amplitude (µV). g. Minimum amplitude (µV). h. Relative power. Data are expressed as median and interquartile range. DMSO: Dimethyl-sulfoxide; PISE: Pyridostigmine-induced Status Epilepticus; SVPA: Sodium Valproate; BP: Blood Pressure. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
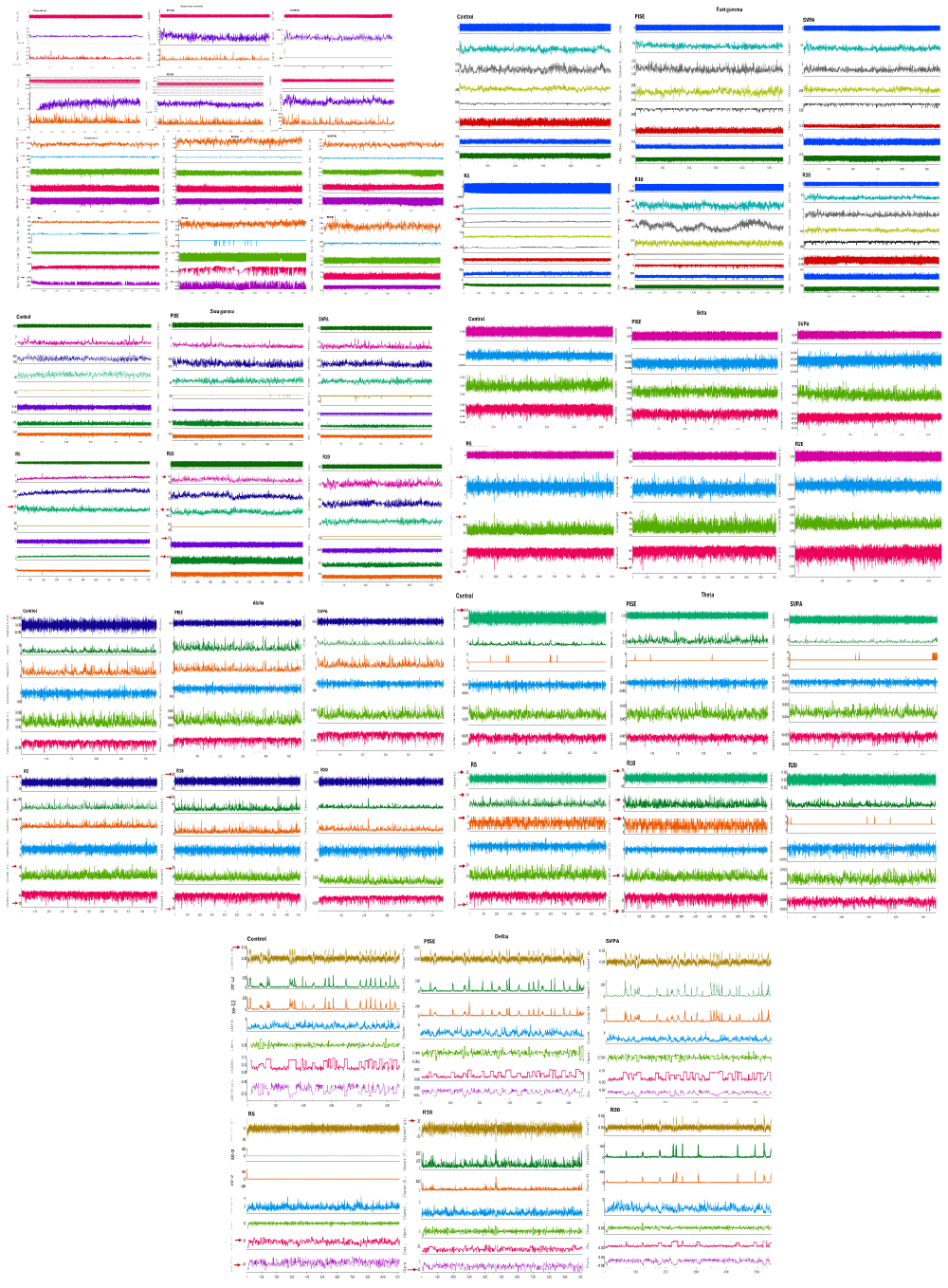
Figure 10: Sample of EEG of adult male Wistar albino rats of a. Controls. b. PISE. c. SVPA. d. R5. e. R10. f. R20. EEG was done for sedated rats with closed eyes using the data acquisition system PowerLab 4/30 (ML866, ADInstruments, Australia); Animal BioAmp (ML136, ADInstruments, Australia) and Lab Chart software v8.2, using subdermal scalp needle electrodes (MLA1203; ADInstruments, Australia) inserted according to the manufacturer’s brochure (ADInstruments, Australia). PISE: Pyridostigmine-induced Status Epilepticus; SVPA: Sodium Valproate; R5: Low dose ramipril (5 mg/kg); R10: Moderate dose ramipril (10 mg/kg); R20: High dose ramipril (20 mg/kg).
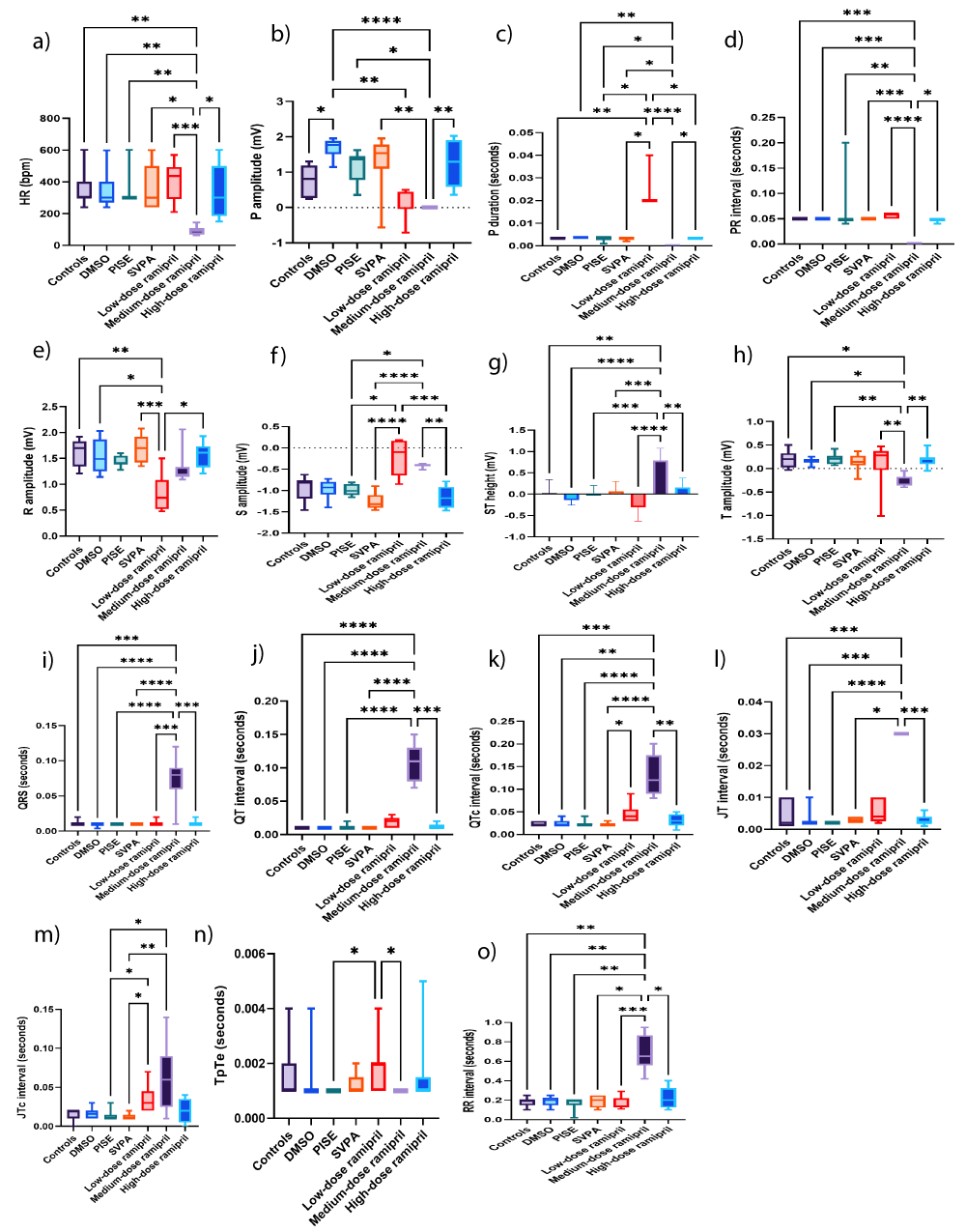
R10 abolished P wave, caused bradycardia, and prolonged QRS and QTc
Compared to controls, neither PISE nor SVPA significantly altered ECG. The changes in BP in absence of variations in heart rate support that the BP changes might occur at distinct brain areas from those involved in heart rate, so that hypotension might occur, even in presence of increased heart rate [77]. In this study, the lack of significant heart rate and diastolic BP variabilities mimicked those trigerred by electrical stimulation of medial prefrontal cortex Brodmann area 25 in patients [78]. The lack of significant ECG changes seemed reassuring and complying with the claimed cardioprotective effect of SVPA in patients with epilepsy [79]. The potential of SVPA to trigger adverse cardiac events owing to the induction of weight gain and metabolic disturbances [80] does not apply herein, given the adopted acute model and SVPA single dosing regimen. Notably, some studies found no association between long-term use of SVPA and cardiac adverse events in new cases of status epilepticus as well as in children with status epilepticus using 5-30 mg/kg doses, as detected by ECG and echocardiography [81,82]. It is worth mentioning that adverse cardiac events could occur in presence of uncontrolled seizures [24], rationalizing the SVPA-associated mortality rate similar to PISE, despite rescue attempts.
R5 prolonged P duration, indicating reduced inter-atrial conductivity, also considered a poor prognostic factor in stroke [83], while reducing R amplitude, indicating poor R wave progression, which was considered a poor prognostic factor in terms of survival [84]. R10 abolished P wave, induced ST elevation and tall T wave, denoting myocardial ischemia, precipitated bradycardia and prolonged QRS, QTc, and RR intervals, denoting heart block. In uncontrolled epilepsy, the risk of cardiovascular disorders, including myocardial infarction and sudden cardiac death is high owing to hypoxia during seizure attacks [85]. The P wave changes associated with R10 suggest atrial fibrillation or flutter, or otherwise P wave and PR interval could be hidden in the prolonged QRS. The absence of P wave in presence of bradycardia could reflect sinus dysfunction, one of the central autonomic changes during interictal episodes [86]. Instead, sinus dysfunction might trigger seizures, secondary to reduced cerebral blood flow [87]. Our findings showed evidence of bradycardia and atrial fibrillation among common cardiovascular adverse reactions encountered in seizures [88]. Peri-ictal bradyarrhythmia, including AV block up to asystole, might occur when bilateral seizure activity is evident on EEG or it might accompany parasympathetic activation, especially with temporal lobe epilepsy [89,90]. QT prolongation, another ECG abnormality recorded during seizures, was also observed in the post-ictal period in this study [91]. Changes in heart rate, P amplitude, P duration, PR interval, R amplitude, S amplitude, ST height, T amplitude, QRS, QT, QTc, JT, JTc, TpTe, and RR intervals are illustrated in Figures 11a-o, respectively. Figure 12 illustrates ECG recordings of the studied groups.
Figure 11: ECG of adult male Wistar albino rats (N = 63). a. HR (bpm). b. P amplitude (mV). c. P duration (seconds). d. PR interval (seconds). e. R amplitude (mV). f. S amplitude. Data are expressed as median and interquartile range. g. ST height (mV). Data are expressed as mean ± SD. h. T amplitude (mV). i. QRS interval (seconds). j. QT (mV). k. QTc (mV). l. JT (mV). m. JTc (mV). n. TpTe (seconds). o. RR interval (seconds). Data are expressed as median and interquartile range. DMSO: Dimethyl-sulfoxide; PISE: Pyridostigmine-induced Status Epilepticus; SVPA: Sodium Valproate; TpTe: T end-Tpeak; QTc: Heart rate-corrected QT interval; JTc: heart rate-corrected JT interval; HR: Heart Rate; bpm: Beat per minute. p ˂ 0.05 is considered statistically significant. * p ˂ 0.05, ** p ˂ 0.01, *** p ˂ 0.001, and **** p ˂ 0.0001.
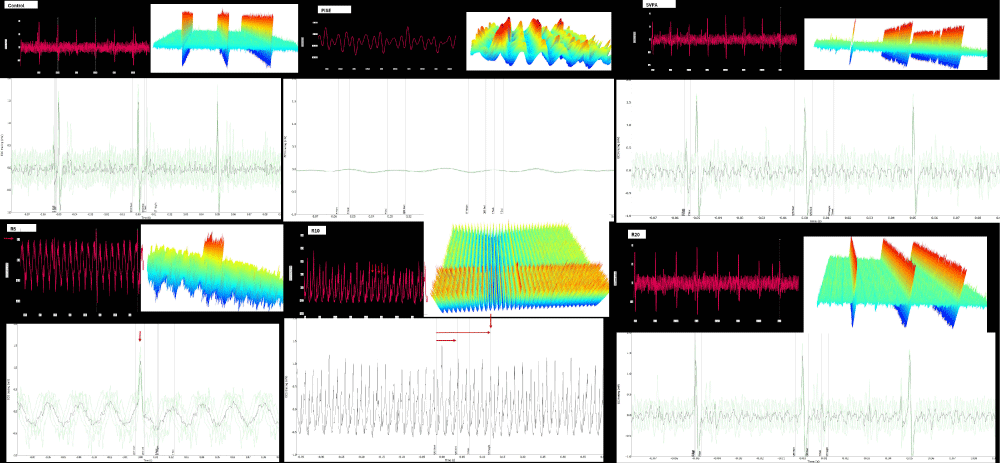
Figure 12: Sample of ECG of adult male Wistar albino rats of a. Controls. b. PISE. c. SVPA. d. R5. e. R10. f. R20. ECG was done for sedated rats with closed eyes using the data acquisition system PowerLab 4/30 (ML866, ADInstruments, Australia) according to the manufacturer’s brochure (ADInstruments, Australia). Each figure consists of 3 panels: upper left: Actual ECG tracing, upper right: waterfall plot, and lower: averaging of beats over 1 second. PISE: pyridostigmine-induced status epilepticus; SVPA: Sodium Valproate; R5: low dose ramipril (5 mg/kg); R10: moderate dose ramipril (10 mg/kg); R20: high dose ramipril (20 mg/kg).
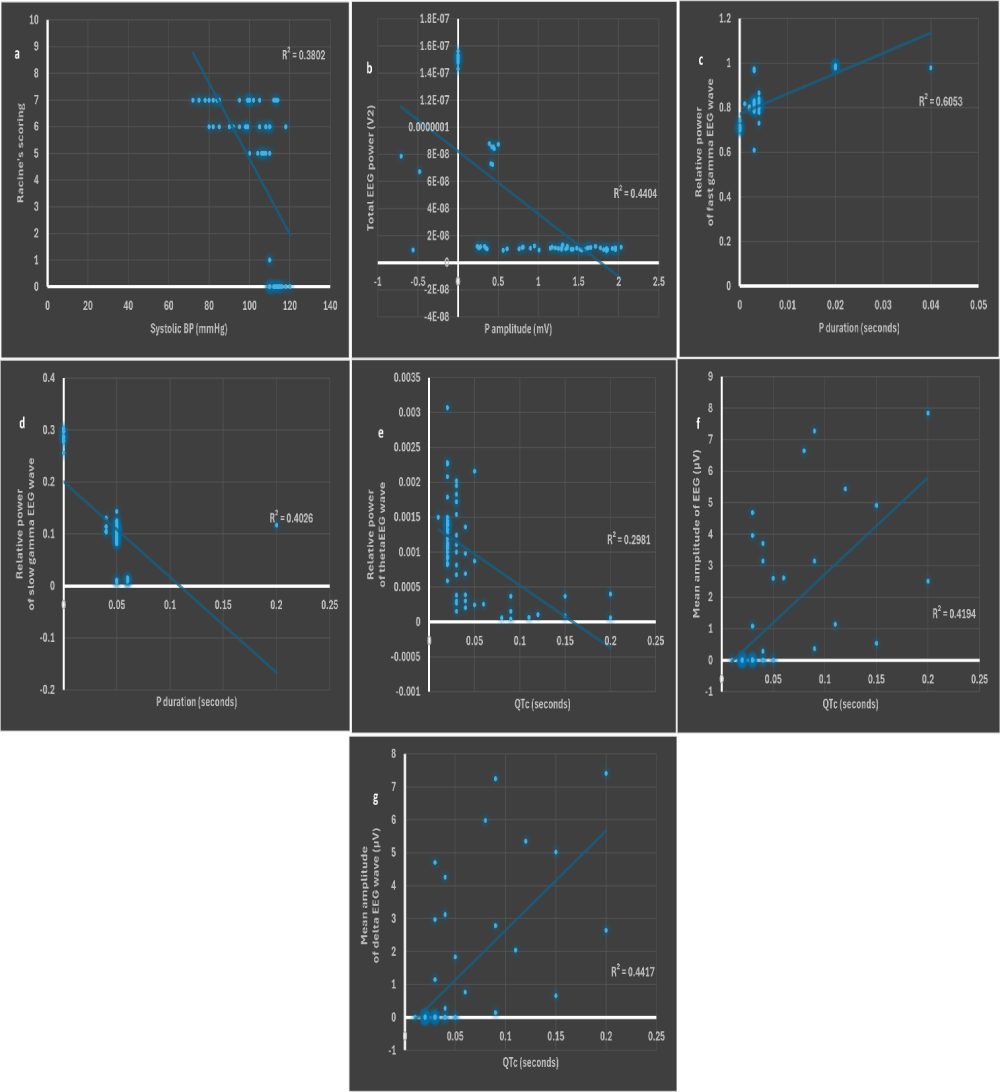
Systolic BP was strongly correlated to Racine’s scoring, while P amplitude and duration as well as QTc were strongly correlated to EEG waves
Only systolic BP was negatively correlated to Racine’s scoring (r = -0.67, p < 0.001) (Figure 13a). A negative correlation between systolic BP and Racine’s scoring could highlight the implication of autonomic dysfunction in PISE model [92].
P amplitude was negatively correlated to the total EEG power (r = -0.62, p < 0.01) (Figure 13b) emphasized that sinus dysfunction might trigger seizures, possibly secondary to reduced cerebral blood flow [87].
P duration was positively correlated to the relative power of fast gamma (r = 0.63, p < 0.01) (Figure 13c), while being negatively correlated to the relative power of slow gamma (r = -0.77, p < 0.0001) (Figure 13d), perhaps substantiating the contribution of cerebral ischemia to epileptogenesis.
Figure 13: Scatter plots of a. systolic BP (mmHg) and Racine’s scoring. b. P amplitude (mV) and total power of source EEG wave (V2). c. P duration (seconds) and relative power of fast gamma EEG wave. d. P duration (seconds) and relative power of slow gamma EEG wave. e. QTc (seconds) and relative power of theta EEG wave. f. QTc (seconds) and mean amplitude of source EEG wave (µV). g. QTc (seconds) and mean amplitude of delta EEG wave (µV). BP: blood pressure. p ˂ 0.05 is considered statistically significant. * r > 0.6 is considered as strong.
QTc was negatively correlated to the relative power of theta wave (r = -0.68, p < 0.01) (Figure 13e), possibly highlighting impaired cognition, the latter might complicate epileptogenic activity [93,94]. Conversely, QTc was positively correlated to the mean amplitudes of source and delta waves (r = 0.64 and r = 0.63, respectively, p < 0.01) (Figures 13f and g). The prolonged QTc denoting heart block might have contributed to cerebral ischemia and subsequent epileptogenesis. Other correlations are illustrated in Figure 14.
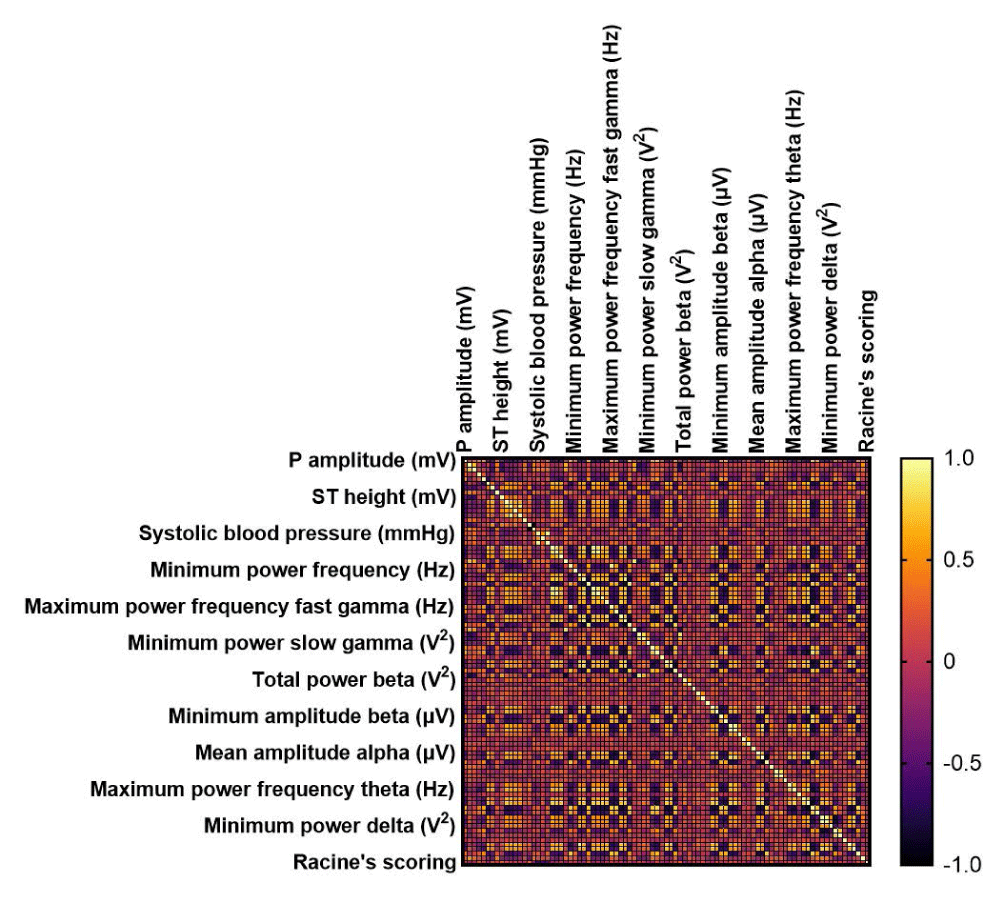
Figure 14: Correlation matrix among the studied groups between Racine’s scoring, blood pressure, EEG, and ECG parameters. EEG: Electroencephalography; ECG: Electrocardiography. p ˂ 0.05 is considered statistically significant.
Limitations of this study
All assessments apart from Racine’s scoring could not be made during the acute severe seizures but rather following 24-hour recovery period.
R5 reduced the severity of status epilepticus induced by pyridostigmine; however, this was accompanied by epileptogenic activity as detected 24 hours later. Neither SVPA, R10 nor R20 yielded anticonvulsant activities in PISE model. The EEG changes noticed with SVPA and R20 highlighted reduced cortical excitability, in contrast to R5 and R10, which demonstrated epileptogenic activity, and possibly some cognitive enhancement attributed to R5. Ramipril exerted dose-dependent reductions in BP, which may have contributed to epileptogenesis . Despite the therapeutic failure of SVPA in PISE model, SVPA could be regarded as safe in terms of cardiac electrophysiology. As with ramipril-induced EEG changes, the ECG recordings, especially with R10, indicated arrhythmogenic and ischemic activities, some of which were strongly correlated to some EEG aspects of epileptogenesis, potentially contributing to intractable seizure, also triggering some EEG aspects of cognitive dysfunction. Ramipril monotherapy, particularly at high doses, should be avoided in the PISE model due to its therapeutic failure and potential to exacerbate epileptogenesis through adverse hypotension and cardiac events. Further research work using other models of epilepsy is warranted.
- World Health Organization. Epilepsy 2024 [updated 7 February 2024; cited 2024 8 July]. Available from: https://www.who.int/news-room/fact-sheets/detail/epilepsy
- Vezzani A, Scharfman HE. Epileptogenesis. In: Jasper's Basic Mechanisms of the Epilepsies. 5th ed. Oxford University Press; 2024.
- Xu C, Wang Y, Chen Z. Novel mechanism, drug target and therapy in epilepsy. Neurosci Bull. 2024;40(5):561-3. Available from: https://doi.org/10.1007/s12264-024-01215-0
- Dirican AC, Mutluay B, Eren F, Ataklı HD. Importance of long-term EEG in seizure-free patients with normal routine EEG. Arch Epilepsy. 2023;29(3):91-5. Available from: https://archepilepsy.org/articles/importance-of-long-term-eeg-in-seizure-free-patients-with-normal-routine-eeg/doi/ArchEpilepsy.2023.23078
- Janiukstyte V, Owen TW, Chaudhary UJ, Diehl B, Lemieux L, Duncan JS, et al. Normative brain mapping using scalp EEG and potential clinical application. Sci Rep. 2023;13(1):13442. Available from: https://www.nature.com/articles/s41598-023-39700-7
- Vorderwülbecke BJ, Wandschneider B, Weber Y, Holtkamp M. Genetic generalized epilepsies in adults—challenging assumptions and dogmas. Nat Rev Neurol. 2022;18(2):71-83. Available from: https://doi.org/10.1038/s41582-021-00583-9
- Sirven JI. Evaluation and management of drug-resistant epilepsy 2024 [updated 19 December 2023; cited 2024 8 July]. Available from: https://www.uptodate.com/contents/evaluation-and-management-of-drug-resistant-epilepsy
- Trinka E, Leitinger M. Management of status epilepticus, refractory status epilepticus, and super-refractory status epilepticus. Continuum (Minneap Minn). 2022;28(2):559-602. Available from: https://doi.org/10.1212/con.0000000000001103
- Ramos AJ. Brain angiotensin system: a new promise in the management of epilepsy? Clin Sci (Lond). 2021;135(6):725-30. Available from: https://doi.org/10.1042/cs20201296
- Chang KC, Lin CH, Huang JA. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers is associated with a reduced risk of poststroke epilepsy in patients with ischemic stroke. J Am Heart Assoc. 2024;13(17):e035438. Available from: https://doi.org/10.1161/jaha.124.035438
- Rusek M, Czuczwar SJ. A review of clinically significant drug-drug interactions involving angiotensin II receptor antagonists and antiepileptic drugs. Expert Opin Drug Metab Toxicol. 2020;16(6):507-15. Available from: https://doi.org/10.1080/17425255.2020.1763955
- Rathi V, Sagi SS, Yadav AK, Kumar M, Varshney R. Quercetin prophylaxis protects the kidneys by modulating the renin-angiotensin-aldosterone axis under acute hypobaric hypoxic stress. Sci Rep. 2024;14(1):7617. Available from: https://doi.org/10.1038/s41598-024-58134-3
- Booker HE, Goodfriend TL, Tewksbury DA. Plasma renin concentration and phenobarbital levels in patients with epilepsy. Clin Pharmacol Ther. 1979;26(6):715-7. Available from: https://doi.org/10.1002/cpt1979266715
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, et al. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 2006;31(6):1123-34. Available from: https://doi.org/10.1038/sj.npp.1300921
- Almeida SS, Naffah-Mazzacoratti MdG, Guimarães PB, Wasinski F, Pereira FEG, Canzian M, et al. Carbamazepine inhibits angiotensin I-converting enzyme, linking it to the pathogenesis of temporal lobe epilepsy. Transl Psychiatry. 2012;2(3):e93. Available from: https://doi.org/10.1038/tp.2012.21
- Łukawski K, Czuczwar SJ. Understanding mechanisms of drug resistance in epilepsy and strategies for overcoming it. Expert Opin Drug Metab Toxicol. 2021;17(9):1075-90. Available from: https://doi.org/10.1080/17425255.2021.1959912
- Łukawski K, Raszewski G, Czuczwar SJ. Interactions of aliskiren, a direct renin inhibitor, with antiepileptic drugs in the test of maximal electroshock in mice. Eur J Pharmacol. 2018;819:108-13. Available from: https://doi.org/10.1016/j.ejphar.2017.11.037
- Atanasova D, Tchekalarova J, Ivanova N, Nenchovska Z, Pavlova E, Atanassova N, et al. Losartan suppresses the kainate-induced changes of angiotensin AT1 receptor expression in a model of comorbid hypertension and epilepsy. Life Sci. 2018;193:40-6. Available from: https://doi.org/10.1016/j.lfs.2017.12.006
- Ivanova N, Tchekalarova J. The potential therapeutic capacity of inhibiting the brain renin-angiotensin system in the treatment of co-morbid conditions in epilepsy. CNS Drugs. 2019;33(11):1101-12. Available from: https://doi.org/10.1007/s40263-019-00678-4
- Subramanian D, Ayus JC. Case report: severe symptomatic hyponatremia associated with lisinopril therapy. Am J Med Sci. 1992;303(3):177-9. Available from: https://doi.org/10.1097/00000441-199203000-00009
- De Sarro G, Di Paola ED, Gratteri S, Gareri P, Rispoli V, Siniscalchi A, et al. Fosinopril and zofenopril, two angiotensin-converting enzyme (ACE) inhibitors, potentiate the anticonvulsant activity of antiepileptic drugs against audiogenic seizures in DBA/2 mice. Pharmacol Res. 2012;65(3):285-96. Available from: https://doi.org/10.1016/j.phrs.2011.11.005
- Tastemur Y, Gumus E, Ergul M, Ulu M, Akkaya R, Ozturk A, et al. Positive effects of angiotensin-converting enzyme (ACE) inhibitor, captopril, on pentylenetetrazole-induced epileptic seizures in mice. Trop J Pharm Res. 2020;19(3):637-43. Available from: http://dx.doi.org/10.4314/tjpr.v19i3.26
- Fialho GL, Miotto R, Cavagnollo MT, Melo HM, Wolf P, Walz R, et al. The epileptic heart: Cardiac comorbidities and complications of epilepsy. Atrial and ventricular structure and function by echocardiography in individuals with epilepsy–From clinical implications to individualized assessment. Epilepsy Behav Rep. 2024:100668. Available from: https://doi.org/10.1016/j.ebr.2024.100668
- Liu Z, Thergarajan P, Antonic‐Baker A, Chen Z, Sparks PB, Lannin NA, et al. Cardiac structural and functional abnormalities in epilepsy: A systematic review and meta‐analysis. Epilepsia Open. 2023;8(1):46-59. Available from: https://doi.org/10.1002/epi4.12692
- Ha FJ, Chong T, Cook MJ, Paratz ED. Epilepsy and cardiac arrhythmias: a state-of-the-art review. Clin Electrophysiol. 2025;11(1):217-29. Available from: https://doi.org/10.1016/j.jacep.2024.09.034
- Mazzola L, Mauguière F, Chouchou F. Central control of cardiac activity as assessed by intra-cerebral recordings and stimulations. Neurophysiol Clin. 2023;53(2):102849. Available from: https://doi.org/10.1016/j.neucli.2023.102849
- Rossi KC, Gursky JM, Pang TD, Dhamoon MS. Seizures and status epilepticus may be risk factor for cardiac arrhythmia or cardiac arrest across multiple time frames. Epilepsy Behav. 2021;120:107998. Available from: https://doi.org/10.1016/j.yebeh.2021.107998
- Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet. 2004;364(9452):2212-9. Available from: https://doi.org/10.1016/s0140-6736(04)17594-6
- Zheng Z, Chen H, Chen Y, Tan X. Causal association between epilepsy and its DNA methylation profile and atrial fibrillation. Heart Rhythm. 2024. Available from: https://doi.org/10.1016/j.hrthm.2024.09.008
- Wang J, Huang P, Yu Q, Lu J, Liu P, Yang Y, et al. Epilepsy and long-term risk of arrhythmias. Eur Heart J. 2023;44(35):3374-82. Available from: https://doi.org/10.1093/eurheartj/ehad523
- Leitinger M, Trinka E, Giovannini G, Zimmermann G, Florea C, Rohracher A, et al. Epidemiology of status epilepticus in adults: a population-based study on incidence, causes, and outcomes. Epilepsia. 2019;60(1):53-62. Available from: https://doi.org/10.1111/epi.14607
- Giussani G, Falcicchio G, La Neve A, Costagliola G, Striano P, Scarabello A, et al. Sudden unexpected death in epilepsy: A critical view of the literature. Epilepsia Open. 2023;8(3):728-57. Available from: https://doi.org/10.1002/epi4.12722
- Bardai A, Blom MT, Van Noord C, Verhamme KM, Sturkenboom MC, Tan HL. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart. 2015;101(1):17-22. Available from: https://doi.org/10.1136/heartjnl-2014-305664
- Vitorović-Todorović MD, Vujatović-Velimirov T. The reversible inhibitors of acetylcholinesterase as pretreatment options against nerve agents’ intoxications. In: Sensing of Deadly Toxic Chemical Warfare Agents, Nerve Agent Simulants, and their Toxicological Aspects. 2023:503-28. Available from: https://doi.org/10.1016/B978-0-323-90553-4.00010-X
- Herkert N, Thiermann H, Worek F. In vitro kinetic interactions of pyridostigmine, physostigmine and soman with erythrocyte and muscle acetylcholinesterase from different species. Toxicol Lett. 2011;206(1):41-6. Available from: https://doi.org/10.1016/j.toxlet.2011.03.004
- Wu P, Hong S, Zhong M, Guo Y, Chen H, Jiang L. Effect of sodium valproate on cognitive function and hippocampus of rats after convulsive status epilepticus. Med Sci Monit. 2016;22:5197. Available from: https://doi.org/10.12659/msm.898859
- Vittalrao AM, KG MR. Cognitive enhancing activities of coenzyme Q10, ramipril, and vinpocetine through modulating neuroinflammatory response and oxidative damage inflicted by acute REM sleep deprivation in rats. Biomed Pharmacol J. 2023;16(4):2021-30. Available from: https://dx.doi.org/10.13005/bpj/2778
- Romoli M, Mazzocchetti P, D'Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, et al. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019;17(10):926-46. Available from: https://doi.org/10.2174/1570159x17666181227165722
- Walton NY, Treiman DM. Lorazepam treatment of experimental status epilepticus in the rat: relevance to clinical practice. Neurology. 1990;40(6):990-4. Available from: https://doi.org/10.1212/wnl.40.6.990
- Carpenter's exotic animal formulary. 6th ed: Elsevier; 2022 19 August 2022.
- Patel S, Meldrum BS, Fine A. Susceptibility to pilocarpine-induced seizures in rats increases with age. Behav Brain Res. 1988;31(2):165-7. Available from: https://doi.org/10.1016/0166-4328(88)90019-8
- Abdelmissih S, Abdelgwad M, Ali DME, Negm MSI, Eshra MA, Youssef A. High-dose Agomelatine Combined with Haloperidol Decanoate Improves Cognition, Downregulates MT2, Upregulates D5, and Maintains Krüppel-like Factor 9 But Alters Cardiac Electrophysiology. J Pharmacol Exp Ther. 2024;390(1):125-45. Available from: https://doi.org/10.1124/jpet.123.002087
- University UfLAM-M. Guidelines on anesthesia and analgesia in rats 2023 [cited 2024 12 July 2024]. Available from: https://az.research.umich.edu/animalcare/guidelines/guidelines-anesthesia-and-analgesia-rats
- Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73. Available from: https://doi.org/10.1016/j.seizure.2009.11.005
- Silberman JT, TA. Carbamate toxicity 2024 22 July 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482183/
- Skiba A, Kozioł E, Luca SV, Budzyńska B, Podlasz P, Van Der Ent W, et al. Evaluation of the Antiseizure Activity of Endemic Plant Halfordia kendack Guillaumin and Its Main Constituent, Halfordin, on a Zebrafish Pentylenetetrazole (PTZ)-Induced Seizure Model. Int J Mol Sci. 2023;24(3):2598. Available from: https://doi.org/10.3390/ijms24032598
- Kasap Acungil Z, Tayhan SE, Tosun NG, Nacar T. The interactions of resveratrol and sodium valproate on penicillin-induced epilepsy model: electrophysiological and molecular study. Mol Neurobiol. 2024:1-11. Available from: https://doi.org/10.1007/s12035-024-04502-z
- Ji Z-Y, Huang Y-Q, He W-Z. Sodium valproate combined with topiramate vs. sodium valproate alone for refractory epilepsy: a systematic review and meta-analysis. Front Neurol. 2022;12:794856. Available from: https://doi.org/10.3389/fneur.2021.794856
- Chaney LA, Rockhold RW, Wineman RW, Hume AS. Anticonvulsant-resistant seizures following pyridostigmine bromide (PB) and N, N-diethyl-m-toluamide (DEET). Toxicol Sci. 1999;49(2):306-11. Available from: https://doi.org/10.1093/toxsci/49.2.306
- Tchekalarova J, Georgiev V. Angiotensin peptides modulatory system: how is it implicated in the control of seizure susceptibility? Life Sci. 2005;76(9):955-70. Available from: https://doi.org/10.1016/j.lfs.2004.10.012
- Nguyen P, Brewster AL. Who Dunnit? Angiotensin, Inflammation, or Complement: Unresolved. Epilepsy Curr. 2023;23(2):133-5. Available from: https://doi.org/10.1177/15357597221150057
- Dong X, Fan J, Lin D, Wang X, Kuang H, Gong L, et al. Captopril alleviates epilepsy and cognitive impairment by attenuation of C3-mediated inflammation and synaptic phagocytosis. J Neuroinflammation. 2022;19(1):226. Available from: https://doi.org/10.1186/s12974-022-02587-8
- Minano F, Serrano J, Sancibrian M, Serrano M. Effect of peptidyl‐dipeptidase inhibitors in experimental convulsions in mice. Fundam Clin Pharmacol. 1987;1(2):77-83. Available from: https://doi.org/10.1111/j.1472-8206.1987.tb00547.x
- Sowden N, Booth C, Kaye G. Syncope, epilepsy and ictal asystole: a case series and narrative review. Heart Lung Circ. 2022;31(1):25-31. Available from: https://doi.org/10.1016/j.hlc.2021.07.003
- Clemens B. Valproate decreases EEG synchronization in a use-dependent manner in idiopathic generalized epilepsy. Seizure. 2008;17(3):224-33. Available from: https://doi.org/10.1016/j.seizure.2007.07.005
- Ibrahim MS, Kamat SR, Shamsuddin S. The role of brain wave activity by electroencephalogram (EEG) in assessing cognitive skills as an indicator for driving fatigue: A review. Malaysian J Compos Sci Manuf. 2023;11(1):19-31. Available from: https://akademiabaru.com/submit/index.php/mjcsm/article/view/4723
- Lewis C, Deshpande A, Tesar GE, Dale R. Valproate-induced hyperammonemic encephalopathy: a brief review. Curr Med Res Opin. 2012;28(6):1039-42. Available from: https://doi.org/10.1185/03007995.2012.694362
- Puris E, Fricker G, Gynther M. Targeting transporters for drug delivery to the brain: can we do better? Pharm Res. 2022;39(7):1415-55. Available from: https://doi.org/10.1007/s11095-022-03241-x
- Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood–brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023;8(1):217. Available from: https://doi.org/10.1038/s41392-023-01481-w
- Chvojka J. The Significance of High-Frequency Oscillations in Understanding Ictogenesis and Functional Organization of Epileptogenic Tissue: Czech Technical University; 2024.
- Sato Y, Tsuji Y, Yamazaki M, Fujii Y, Shirasawa A, Harada K, et al. Interictal high gamma oscillation regularity as a marker for presurgical epileptogenic zone localization. Oper Neurosurg. 2022;23(2):164-73. Available from: https://doi.org/10.1227/ons.0000000000000245
- Shi W, Shaw D, Walsh KG, Han X, Eden UT, Richardson RM, et al. Spike ripples localize the epileptogenic zone best: an international intracranial study. Brain. 2024;147(7):2496-506. Available from: https://doi.org/10.1093/brain/awae037
- De Stefano P, Carboni M, Marquis R, Spinelli L, Seeck M, Vulliemoz S. Increased delta power as a scalp marker of epileptic activity: a simultaneous scalp and intracranial electroencephalography study. Eur J Neurol. 2022;29(1):26-35. Available from: https://doi.org/10.1111/ene.15106
- Benbadis S, Beniczky S, Bertram E, MacIver S, Moshé SL. The role of EEG in patients with suspected epilepsy. Epileptic Disord. 2020;22(2):143-55. Available from: https://doi.org/10.1684/epd.2020.1151
- Pottkämper JC, Verdijk JP, Stuiver S, Aalbregt E, Schmettow M, Hofmeijer J, et al. Seizure duration predicts postictal electroencephalographic recovery after electroconvulsive therapy-induced seizures. Clin Neurophysiol. 2023;148:1-8. Available from: https://doi.org/10.1016/j.clinph.2023.01.008
- Chauhan M, PJ, Ahmad F. Ramipril 2024 06 December 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/30725804/
- Lundqvist M, Miller EK, Nordmark J, Liljefors J, Herman P. Beta: bursts of cognition. Trends Cogn Sci. 2024;28(7):662-676. Available from: https://doi.org/10.1016/j.tics.2024.03.010
- VanRullen R, Koch C. Competition and selection during visual processing of natural scenes and objects. J Vis. 2003;3(1):75-85. Available from: https://doi.org/10.1167/3.1.8
- Tan E, Troller-Renfree SV, Morales S, Buzzell GA, McSweeney M, Antúnez M, et al. Theta activity and cognitive functioning: Integrating evidence from resting-state and task-related developmental electroencephalography (EEG) research. Dev Cogn Neurosci. 2024;67:101404. Available from: https://doi.org/10.1016/j.dcn.2024.101404
- Yang W, Luo H, Ma Y, Si S, Zhao H. Effects of antihypertensive drugs on cognitive function in elderly patients with hypertension: a review. Aging Dis. 2021;12(3):841-851. Available from: https://doi.org/10.14336/ad.2020.1111
- Dobrowolski C, Barraclough M, Su J, Tanic M, Bingham K, Ruttan L, et al. Centrally acting ACE inhibitor (cACEi) and angiotensin receptor blocker (cARB) use and cognitive dysfunction in patients with SLE. Lupus Sci Med. 2023;10(2):e000923. Available from: https://doi.org/10.1136/lupus-2023-000923
- Caravaglios G, Muscoso E, Blandino V, Di Maria G, Gangitano M, Graziano F, et al. EEG resting-state functional networks in amnestic mild cognitive impairment. Clin EEG Neurosci. 2023;54(1):36-50. Available from: https://doi.org/10.1177/15500594221110036
- Martin T, Kero K, Požar R, Giordani B, Kavcic V. Mild cognitive impairment in African Americans is associated with differences in EEG theta/beta ratio. J Alzheimers Dis. 2023;94(1):347-57. Available from: https://doi.org/10.3233/jad-220981
- Villacres JE, Riveira N, Kim S, Colgin LL, Noebels JL, Lopez AY. Abnormal patterns of sleep and waking behaviors are accompanied by neocortical oscillation disturbances in an Ank3 mouse model of epilepsy-bipolar disorder comorbidity. Transl Psychiatry. 2023;13(1):403. Available from: https://doi.org/10.1038/s41398-023-02700-2
- Ricci L, Assenza G, Pulitano P, Simonelli V, Vollero L, Lanzone J, et al. Measuring the effects of first antiepileptic medication in Temporal Lobe Epilepsy: Predictive value of quantitative-EEG analysis. Clin Neurophysiol. 2021;132(1):25-35. Available from: https://doi.org/10.1016/j.clinph.2020.10.020
- Cuesta P, Ochoa-Urrea M, Funke M, Hasan O, Zhu P, Marcos A, et al. Gamma band functional connectivity reduction in patients with amnestic mild cognitive impairment and epileptiform activity. Brain Commun. 2022;4(2):fcac012. Available from: https://doi.org/10.1093/braincomms/fcac012
- Akyüz E, Üner AK, Köklü B, Arulsamy A, Shaikh MF. Cardiorespiratory findings in epilepsy: A recent review on outcomes and pathophysiology. J Neurosci Res. 2021;99(9):2059-73. Available from: https://doi.org/10.1002/jnr.24861
- Tavares R, Corrêa FMdA, Resstel L. Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J Neurosci Res. 2009;87(11):2601-7. Available from: https://doi.org/10.1002/jnr.22070
- Abaseynejad F, Akrami R, Mohebbati R, Sehab Negah S, Mohammad-Zadeh M. The effect of sodium valproate on cardiovascular responses in pentylenetetrazol kindling model of epilepsy. Biomed J Sci Tech Res. 2022;42:33592-6. Available from: https://biomedres.us/fulltexts/BJSTR.MS.ID.006746.php
- Jia L, Verkerk AO, Tan HL. The anti-epileptic drugs lamotrigine and valproic acid reduce the cardiac sodium current. Biomedicines. 2023;11(2):477. Available from: https://doi.org/10.3390/biomedicines11020477
- Radgoudarzi M, Vafaee-Shahi M, Naderi F. Effect of sodium valproate treatment on the cardiac index in new cases with status epilepticus. Open Neurol J. 2021;15(1):59-64. Available from: https://doi.org/10.2174/1874205X02115010059
- Kamani A, Omidi H, Dorreh F, Shariatmadari F, Ghandi Y. Effect of sodium valproate on cardiac function in epileptic children by tissue doppler echocardiography. Acta Med Iran. 2020:472-8. Available from: https://acta.tums.ac.ir/index.php/acta/article/view/8342
- Wu Y, Yang X, Jing J, Meng X, Li Z, Pan Y, et al. Prognostic significance of atrial cardiopathy in patients with acute ischemic stroke. Eur Stroke J. 2023;8(1):183-90. Available from: https://doi.org/10.1177/23969873221126000
- Schröder LC, Holkeri A, Eranti A, Haukilahti MAE, Kerola T, Kenttä TV, et al. Poor R-wave progression as a predictor of sudden cardiac death in the general population and subjects with coronary artery disease. Heart Rhythm. 2022;19(6):952-9. Available from: https://doi.org/10.1016/j.hrthm.2022.02.010
- Verrier RL, Pang TD, Nearing BD, Schachter SC. Epileptic heart: a clinical syndromic approach. Epilepsia. 2021;62(8):1780-9. Available from: https://doi.org/10.1111/epi.16966
- Sasaki R, Osugi N, Nakagawa I. Generalized epilepsy with repetitive sinus pauses following generalized tonic-clonic seizures due to reduced baroreflex sensitivity. Cureus. 2023;15(5):e39392. Available from: https://doi.org/10.7759/cureus.39392
- Patel N, Majeed F, Sule AA. Seizure triggered by sick sinus syndrome. Case Rep. 2017;2017:bcr-2017-222011. Available from: https://doi.org/10.1136/bcr-2017-222011
- Arens AM, Kearney T. Adverse effects of physostigmine. J Med Toxicol. 2019;15:184-91. Available from: https://doi.org/10.1007/s13181-019-00697-z
- Yousif R, Abdulghani MO, Gaber A, El Khayat N, Abo Elnaga Y, Wahid El Din MM. Frequency of peri-ictal apnea and cardiac arrhythmias in epileptic seizures. Egypt J Neurol Psychiatry Neurosurg. 2021;57:1-8. Available from: https://ejnpn.springeropen.com/articles/10.1186/s41983-021-00295-3
- Ouchida S, Parratt K, Nikpour A, Fairbrother G. Syncope vs. Seizure: Ictal Bradycardia and Ictal Asystole. Case Rep Neurol Med. 2024;2024(1):1299282. Available from: https://doi.org/10.1155/2024/1299282
- Stöllberger C, Finsterer J. Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP). Epilepsy Res. 2004;59(1):51-60. Available from: https://doi.org/10.1016/j.eplepsyres.2004.03.008
- Geersing PG, Bulte CS, Viersen VA, Stek ML, Bouwman RA, Boer C, et al. Beat-to-beat hemodynamic monitoring during electroconvulsive therapy. J ECT. 2011;27(3):189-91. Available from: https://doi.org/10.1097/yct.0b013e3182008de5
- Novak A, Vizjak K, Rakusa M. Cognitive impairment in people with epilepsy. J Clin Med. 2022;11(1):267. Available from: https://doi.org/10.3390/jcm11010267
- Sayed NM, Aldin MTK, Ali SE, Hendi AE. Cognitive functions and epilepsy-related characteristics in patients with generalized tonic–clonic epilepsy: a cross-sectional study. Middle East Curr Psychiatry. 2023;30(1):15. Available from: https://mecp.springeropen.com/articles/10.1186/s43045-023-00293-6