More Information
Submitted: November 15, 2024 | Approved: December 02, 2024 | Published: December 03, 2024
How to cite this article: Pervin S, Nasaruddin A, Irfan M, Annamalai L. Sexual Dimorphism in the Length of the Corpus Callosum in Cadaver. J Neurosci Neurol Disord. 2024; 8(2): 126-129. Available from: https://dx.doi.org/10.29328/journal.jnnd.1001104
DOI: 10.29328/journal.jnnd.1001104
Copyright License: © 2024 Pervin S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Corpus callosum; Length; Sexual dimorphism
Sexual Dimorphism in the Length of the Corpus Callosum in Cadaver
Shahnaj Pervin1*, Nasaruddin A2, Irfan M3 and Annamalai L4
1Shahnaj Pervin, Senior Lecturer, School of Medicine, IMU University, Malaysia
2Nasaruddin Bin Abdul Aziz, Professor of Anatomy, University of Cyberjaya, Malaysia
3Mohammed Irfan, Senior Lecturer, School of Medicine, IMU University, Malaysia
4Lakshmi Annamalai, Professor of Anatomy, Management and Science University, Malaysia
*Address for Correspondence: Dr. Shahnaj Pervin, Senior lecturer, Department of Anatomy, School of Medicine, IMU University, 126, Jln Jalil Perkasa 19, Bukit Jalil, 57000 Kuala Lumpur, Federal Territory of Kuala Lumpur, Malaysia, Email: [email protected]
Context: Several texts and literature suggest that corpus callosum may be sexually dimorphic. Previous researchers found that the length of the corpus callosum is larger in males than in females. Reviewing various foreign literature found that the length of the corpus callosum may change in many diseases in Neurology, Neurosurgery, and Psychiatry. So, knowledge of the normal morphological difference of the length of corpus callosum between Bangladeshi males and females is essential for the diagnosis in brain imaging and treatment of those diseases.
Objective: The present study was conducted to provide data on the length of the corpus callosum of our people, which can be used to set a standard measurement for the Bangladeshi population.
Materials and methods: A cross-sectional, descriptive study was done in the Department of Anatomy, Dhaka Medical College, Dhaka, Bangladesh, from July 2009 to June 2010, based on the collection of 60 human brains (male 36 and female 24) from unclaimed dead bodies. The lengths were measured by using digital slide calipers in mm.
Results: The mean length of the corpus callosum in males and females in groups A, B, C & D (grouping in done on age difference) were 68.04 ± 0.99 and 67.03 ± 0.05 mm, 67.50 ± 0.13 and 67.02 ± 0.03 mm and 67.51 ± 0.03 and 67.02 ± 0.03 mm respectively.
Conclusion: Statistically significant differences were found between males and females in all age groups in the length of the corpus callosum.
The corpus callosum is the major commissural fiber of the brain connecting the two cerebral hemispheres [1,2]. Its different parts are the rostrum, genu, body, and splenium [3,4]. It is a bundle of white matter likely to be affected by physiological and pathological changes in the cortical and subcortical regions of the brain [5,6].
Corpus callosum morphology, dimensions, and gender differences have attracted the interest of scientists in recent years due to the increasing number of callosotomies being performed for the treatment of some forms of generalized epilepsy. Surgical transection of the corpus callosum causes a reduction in seizure frequency in epilepsy patients [7,8]. Neurosurgeons’ attention to the corpus callosum has been reawakened due to advanced surgical techniques. Its large size, central location, and widespread connections encourage scientific curiosity and therapeutic considerations. The corpus callosum has become increasingly important to surgeons for approaching structures like lateral ventricles [9,10].
Length of the corpus callosum may vary in different neurological conditions, including increases in type-1 neurofibromatosis patients and decreases in cortical hypogenesis, aging, and alcoholism [11,12]. These conditions are associated with certain psychiatric illnesses such as schizophrenia and autism. It acts as a marker for cortical pathology of neurodegenerative diseases [13-15].
Abnormalities in the Corpus Callosum (CC) length are associated with several neuropsychiatric conditions, making radiological assessment crucial for diagnosis and monitoring. In Autism Spectrum Disorders (ASD), recent studies have shown reduced CC length, particularly in the anterior regions [16,17]. Schizophrenia patients often exhibit CC thinning and length alterations, affecting interhemispheric communication [14]. Multiple Sclerosis (MS) commonly involves CC atrophy, where length measurements help track disease progression [18]. In Alzheimer’s disease, CC atrophy patterns, including length changes, can serve as early biomarkers [19]. Radiologically, understanding normal CC anatomy is essential for early detection of pathological changes, differentiating between various neuropsychiatric conditions, monitoring disease progression, and evaluating treatment effectiveness. Standardized measurements of CC length using advanced MRI techniques have become valuable diagnostic tools, particularly in conditions where subtle changes might indicate disease onset or progression before clinical symptoms become apparent [6].
So, standard normative data on the morphology of corpus callosum is very important for the diagnosis and treatment of such neuropsychiatric diseases.
The study was done on 60 (36 male and 24 female) human brains of Bangladeshi people. Samples were collected from the unclaimed dead bodies that were under examination in the Department of Anatomy and Forensic Medicine, Dhaka Medical College, Dhaka within 24 to 36 hours of death. After collection samples were washed and fixed in 40% formaldehyde solution (origin-Germany) for 15 days.
After fixation, samples were washed in running tap water to eliminate excess formalin and cut carefully in the median plane by using sharp scissors, fine dissecting forceps, and a BP blade along the longitudinal fissure from front to back. The fax cerebri containing superior and inferior sagittal sinus, fornix, septum pellucidum, inter thalamic adhesion, and brain stem were cut step by step thus the hemispheres were divided into two halves. Samples were put into another tray and the morphological measurements of the length of the corpus callosum from both hemispheres were taken.
The samples were divided into four different age groups i.e, Group A (20-29 years), Group B (30-39 years), C (40-49 years), D (50-59 years).
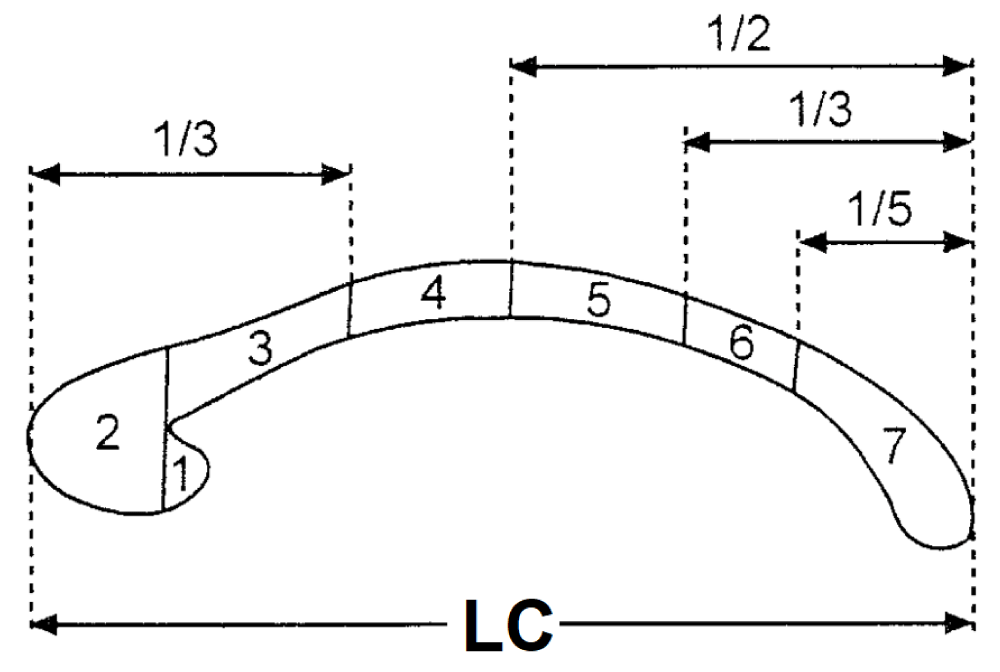
The length (LC) of the corpus callosum is the distance from the anterior-most point of genu to the posterior-most of the splenium4 (Figure 1) The distance from both hemispheres was measured by using digital slide calipers in mm (Photograph 1).
Figure 1: Showing measurement procedure of length of corpus callosum (LC) Corpus Callosum Subregions. 1-rostrum; 2-genu; 3-rostral body; 4-anterior midbody; 5-posterior midbody; 6-isthmus; and 7-splenium. LC is the length of the corpus callosum.
Photograph 1: Measurement of length of corpus callosum by digital slide calipers in mm.
Ethical approval
The study was conducted after the approval of the ethical review committee of Dhaka Medical College. No: DMC/ Ethical/2010/49.
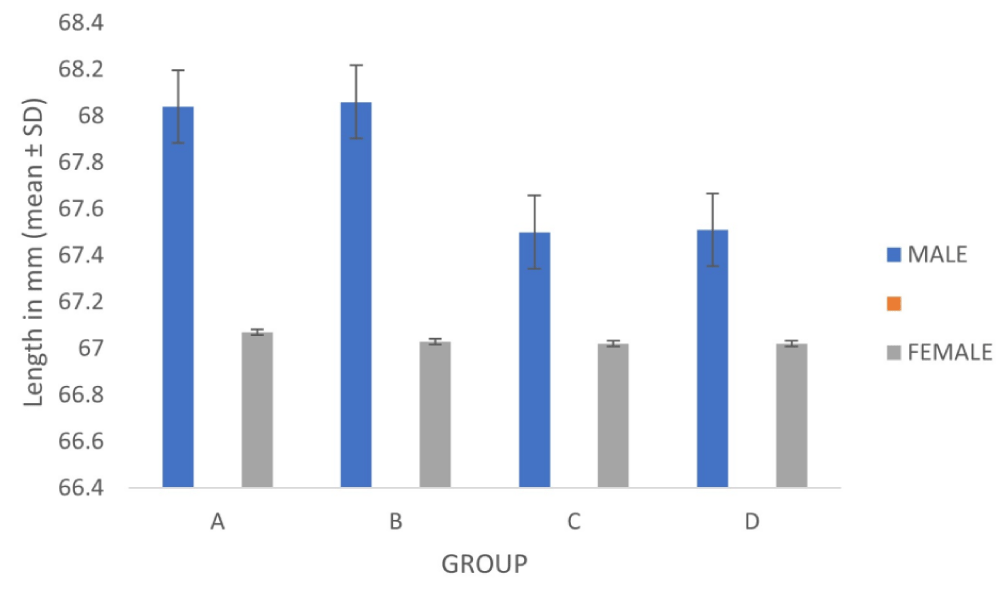
The (mean ± SD) length of the corpus callosum in males and females in groups A, B, C & D were 68.04 ± 1.89 and 67.07 ± 0.21 mm. 68.06 ± 0.99 and 67.03 ± 0.05 mm, 67.50 ± 0.13 and 67.02 ± 0.03 mm and 67.51 ± 0.03 and 67.02 ± 0.03 mm respectively. Statistically significant mean differences in the length of corpus callosum between males and females were found in group A (p < 0.05), group B (p < 0.01), and groups C and D (p < 0.001) (Table 1, Figure 2).
Figure 2: Length of the corpus callosum of males and females in different age groups.
| Table 1: Length of the corpus callosum of males and females in different age groups. | |||
| Age Group | Male (Mean ± SD) (mm) | Female (Mean ± SD) (mm) | p - value |
| Group A (20 to 29 years) | 68.04 ± 1.19 (66.02 - 70.01) n=9 | 67.07 ± 0.21 (66.72 - 67.35) n=8 | < 0.05* |
| Group B (30 to 39 years) | 69.06 ± 0.99 (66.10 - 69.89) n=12 | 67.03 ± 0.05 (66.94 - 67.13) n=8 | < 0.01** |
| Group C (40 to 49 years) | 67.50 ± 0.13 (67.25 - 67.71) n=8 | 67.02 ± 0.03 (66.68 - 67.06) n=5 | < 0.001*** |
| Group D (50 to 59 years) | 67.51 ± 0.03 (67.47 - 67.56) n=7 | 67.02 ± 0.03 (66.99 - 67.05) n=3 | < 0.001*** |
| A vs. B | > 0.50 ns | ||
| A vs. C | > 0.50 ns | ||
| A vs. D | > 0.50 ns | ||
| B vs. C | > 0.50 ns | ||
| B vs. D | > 0.50 ns | ||
| C vs. D | > 0.50 ns | ||
| Comparison between males and females done by unpaired student’s ‘t’ test and comparison between the age group of males and females done by One-way ANOVA ( PostHoc), ns = not significant, */**/*** = significant. Group A (20-29 years), Group B (30-39 years), C (40-49 years), D (50-59 years). |
|||
The (mean ± SD) length of the corpus callosum was 67.59 ± 0.99 mm, 67.45 ± 0.9mm, 67.32 ± 0.26 mm, and 67.36 ± 0.24 mm in Group A, B, C and D respectively. The mean difference between different age groups of this study was statistically not significant. Figure 2 shows the length difference of corpus callosum in males and females in different age groups.
The results of this study are closer to the morphometric analyses by Gupta, T. [7], Patra, et al. 2020 [20], and Almalki, et al. 2024 [21], who found that the length of the corpus callosum was 69.8 mm in males and 68.6 mm in females in their South Asian population study. The present study results also support recent investigations that have documented sexual dimorphism in corpus callosum length [22-26]. The result of the present study is similar to the values reported in the Indian study but varies from the values of the study done on Caucasians [27], and the Japanese population [20,28].
The youngest age group (20 - 29 years) had the longest mean CC length [29]. The longest CC length was found in males aged 30-39 years (68.06 ± 0.99 mm), which aligns with recent findings on age-related corpus callosum development [25].
The values of the present study demonstrated greater corpus callosum length in males compared to females, consistent with recent large-scale morphometric studies [22,30,31].
Statistically significant differences in the length of the corpus callosum between Bangladeshi males and females in different age groups were found. The limitation of the study was conducted with preserved samples, which may cause shrinkage of the viscera. Additionally, measurements may vary compared to those obtained from MRI imaging. For future works, a comparative study of preserved brains and MRI scans in the Bangladeshi population is recommended to determine the differences in measurements between cadaveric brains and MRI scan brains.
- Snell RS. Clinical neuroanatomy. Lippincott Williams & Wilkins; 2010. Available from: https://www.google.co.in/books/edition/Clinical_Neuroanatomy/ABPmvroyrD0C?hl=en
- Shah A, Jhawar S, Goel A, Goel A. Corpus callosum and its connections: a fiber dissection study. World Neurosurg. 2021;151:e1024-e1035. Available from: https://doi.org/10.1016/j.wneu.2021.05.047
- Gray’s Anatomy, 39th edition: the anatomical basis of clinical practice. AJNR Am J Neuroradiol. 2005;26(10):2703–4. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7976199/
- Pervin S, Nurunnabi ASM, Ara S, Haroon K. Morphometry of the splenium of the corpus callosum–a study on 60 cadaveric brains. Bangladesh J Neurosurg. 2021;11(1):30-35. Available from: https://www.banglajol.info/index.php/BJNS/article/view/57990
- Ilayperuma I, Nanayakkara G, Palahepitiya N. Gross anatomical study on the gender differences in the corpus callosum. Galle Med J. 2009;14(1). Available from: https://gmj.sljol.info/articles/10.4038/gmj.v14i1.1167
- Albadawi EA. Microstructural changes in the corpus callosum in neurodegenerative diseases. Cureus. 2024;16(8):e67378. Available from: https://doi.org/10.7759/cureus.67378
- Gupta T, Singh B, Kapoor K, Gupta M, Kochhar S. Normative data of corpus callosal morphology in a North-West Indian population–an autopsy and MRI study. JNMA J Nepal Med Assoc. 2009;48(173):46-51. Available from: https://pubmed.ncbi.nlm.nih.gov/19529058/
- Vaddiparti A, Huang R, Blihar D, Du Plessis M, Montalbano MJ, Tubbs RS, Loukas M. The evolution of corpus callosotomy for epilepsy management. World Neurosurg. 2021;145:455-461. Available from: https://doi.org/10.1016/j.wneu.2020.08.178
- Frazier TW, Hardan AY. A meta-analysis of the corpus callosum in autism. Biol Psychiatry. 2009;66(10):935-941. Available from: https://doi.org/10.1016/j.biopsych.2009.07.022
- Dang DD, Rechberger JS, Leonel LC, Rindler RS, Nesvick CL, Graepel S, et al. Anatomical step-by-step dissection of common approaches to the third ventricle for trainees: surgical anatomy of the anterior transcortical and interhemispheric transcallosal approaches, surgical principles, and illustrative pediatric cases. Acta Neurochir (Wien). 2023;165(9):2421-2434. Available from: https://mayoclinic.elsevierpure.com/en/publications/anatomical-step-by-step-dissection-of-common-approaches-to-the-th
- Caruso PA. Disorders of brain development. Paul A. Caruso, Richard Robertson, Bindu Setty, and Ellen Grant. Magnetic Reson Imaging Brain Spine. 2009;1:194.
- Hofman J, Hutny M, Sztuba K, Paprocka J. Corpus callosum agenesis: an insight into the etiology and spectrum of symptoms. Brain Sci. 2020;10(9):625. Available from: https://doi.org/10.3390/brainsci10090625
- Tabares-Seisdedos R, Rubenstein JL. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: implications for schizophrenia, autism and cancer. Mol Psychiatry. 2009;14(6):563-589. Available from: https://doi.org/10.1038/mp.2009.2
- Piras F, Vecchio D, Kurth F, Piras F, Banaj N, Ciullo V, et al. Corpus callosum morphology in major mental disorders: a magnetic resonance imaging study. Brain Commun. 2021;3(2):fcab100. Available from: https://doi.org/10.1093/braincomms/fcab100
- Khasawneh RR, Abu-El-Rub E, Alzu’bi A, Abdelhady GT, Al-Soudi HS. Corpus callosum anatomical changes in Alzheimer patients and the effect of acetylcholinesterase inhibitors on corpus callosum morphometry. PLoS One. 2022;17(7):e0269082. Available from: https://doi.org/10.1371/journal.pone.0269082
- Badhe S, Nivins S, Kulkarni P, Jose A, Manek D, Badhe S, et al. Abnormal development of the corpus callosum in autism spectrum disorder: an MRI study. Top Magn Reson Imaging. 2024;33(3):e0312. Available from: https://doi.org/10.1097/rmr.0000000000000312
- Chien YL, Chen YJ, Tseng WL, Hsu YC, Wu CS, Tseng WYI, Gau SSF. Differences in white matter segments in autistic males, non-autistic siblings, and non-autistic participants: an intermediate phenotype approach. Autism. 2023;27(4):1036-1052. Available from: https://doi.org/10.1177/13623613221125620
- Sotgiu MA, Piga G, Mazzarello V, Zarbo IR, Carta A, Saderi L, et al. Corpus callosum volumetrics and clinical progression in early multiple sclerosis. Eur Rev Med Pharmacol Sci. 2022;26(1). Available from: https://doi.org/10.26355/eurrev_202201_27772
- Das S, Panigrahi P, Chakrabarti S. Corpus callosum atrophy in detection of mild and moderate Alzheimer’s disease using brain magnetic resonance image processing and machine learning techniques. J Alzheimers Dis Rep. 2021;5(1):771-788. Available from: https://doi.org/10.3233/adr-210314
- Patra A, Singla R, Chaudhary P, Malhotra V. Morphometric analysis of the corpus callosum using cadaveric brain: an anatomical study. Asian J Neurosurg. 2020;15(02):322-327. Available from: https://doi.org/10.4103/ajns.ajns_328_19
- Almalki YE, Basha MAA, Metwally MI, Zeed NA, Nada MG, Alduraibi SK, et al. Validating Brain Tumor Reporting and Data System (BT-RADS) as a diagnostic tool for glioma follow-up after surgery. Biomedicines. 2024;12(4):887. Available from: https://doi.org/10.3390/biomedicines12040887
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30(33):10985-10990. Available from: https://doi.org/10.1523/JNEUROSCI.5122-09.2010
- Welcome SE, Chiarello C, Towler S, Halderman LK, Otto R, Leonard CM. Behavioral correlates of corpus callosum size: anatomical/behavioral relationships vary across sex/handedness groups. Neuropsychologia. 2009;47(12):2427-2435. Available from: https://doi.org/10.1016/j.neuropsychologia.2009.04.008
- Hopkins WD, Phillips KA. Cross-sectional analysis of the association between age and corpus callosum size in chimpanzees (Pan troglodytes). Dev Psychobiol. 2010;52(2):133-141. Available from: https://doi.org/10.1002/dev.20421
- Pasricha N, Sthapak E, Thapar A, Bhatnagar R. Morphometric analysis of age and gender-related variations of corpus callosum by using magnetic resonance imaging: a cross-sectional study. J Clin Diagn Res. 2023;17(6):AC15-AC20. Available from: https://doi.org/10.7860/JCDR/2023/63555.18078
- Al-Hadidi MT, Kalbouneh HM, Ramzy A, Al Sharei A, Badran DH, Shatarat A, et al. Gender and age-related differences in the morphometry of corpus callosum: MRI study. Eur J Anat. 2021;25(1):15-24. Available from: https://eurjanat.com/articles/gender-and-age-related-differences-in-the-morphometry-of-corpus-callosum-mri-study/
- Poleneni SR, Jakka LD, Chandrupatla M, Vinodini L, Ariyanachi K. Morphometry of corpus callosum in south Indian population. Ann Neurosci. 2021;28(3-4):150-155. Available from: https://doi.org/10.1177/09727531211059892
- Lee BY, Sohn JH, Choi MH, Lee SJ, Kim HS, Yang JW, et al. A volumetric study of the corpus callosum in 20s and 40s Korean people. Brain Struct Funct. 2009;213:463-467. Available from: https://doi.org/10.1007/s00429-009-0209-5
- Danielsen VM, Vidal-Piñeiro D, Mowinckel AM, Sederevicius D, Fjell AM, Walhovd KB, et al. Lifespan trajectories of relative corpus callosum thickness: regional differences and cognitive relevance. Cortex. 2020;130:127-141. Available from: https://doi.org/10.1016/j.cortex.2020.05.020
- Chura LR, Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Bullmore ET, Baron-Cohen S. Organizational effects of fetal testosterone on human corpus callosum size and asymmetry. Psychoneuroendocrinology. 2010;35(1):122-132. Available from: https://doi.org/10.1016/j.psyneuen.2009.09.009
- Eliot L, Ahmed A, Khan H, Patel J. Dump the “dimorphism”: Comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci Biobehav Rev. 2021;125:667-697. Available from: https://doi.org/10.1016/j.neubiorev.2021.02.026