More Information
Submitted: November 06, 2024 | Approved: November 11, 2024 | Published: November 12, 2024
How to cite this article: Alex, Gideon S, Oke OO, Ekokojde JW, Gbayisomore TJ, Anene-Ogbe MC, Glory F, et al. Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience Research. J Neurosci Neurol Disord. 2024; 8(2): 106-114. Available from: https://dx.doi.org/10.29328/journal.jnnd.1001102.
DOI: 10.29328/journal.jnnd.1001102
Copyright License: © 2024 Alex, Gideon S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Adult neurogenesis; Neural stem cell; Neuroplasticity; Emerging technologies
Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience Research
Alex, Gideon S1*, Olanrewaju Oluwaseun Oke2, Joy Wilberforce Ekokojde3, Tolulope Judah Gbayisomore4, Martina C. Anene-Ogbe1, Farounbi Glory5 and Joshua Ayodele Yusuf6
1Department of Anatomy, Faculty of Basic Medical Sciences, University of Port Harcourt, East-West Road, PMB 5323 Choba, Rivers State 500102, Nigeria
2Department of Physiology, School of Basic Medical Sciences, Federal University of Technology, Akure, PMB 704, Akure, Ondo State 340106, Nigeria
3Department of Human Anatomy, College of Medicine, Rivers State University, P.M.B 5080, Port Harcourt, Nigeria
4University of Benin Teaching Hospital, P.M.B. 1154, Ugbowo, Benin City, Edo State 300001, Nigeria
5College of Medicine, Lagos State University, PMB 001 Ikeja, Lagos State 100282, Nigeria
6Ladoke Akintola University of Technology, P.M.B 4000, Ogbomoso, Oyo State, Nigeria
*Address for Correspondence: Gideon Sorlelodum Alex, Department of Anatomy, Faculty of Basic Medical Sciences, University of Port Harcourt, East-West Road, PMB 5323 Choba, Rivers State 500102, Nigeria, Email: [email protected]
Background: The study of new neuron formation in the adult brain has sparked controversy and ignited interest among scientists in recent times, these include its occurrence and location in the adult human brain, functional significance, variation in study methods, translation from animal model to human, and ethical challenges involving neural stem cell research.
Aim: To provide a comprehensive understanding of adult neurogenesis, functional significance, and challenges and explore the latest advances in the study of adult neurogenesis.
Methodology: An extensive and systematic search of electronic databases (Medline, Scopus, Web of Science) was conducted using keywords related to adult neurogenesis and techniques involved in its study.
Results: The mechanism of adult neurogenesis was found to occur in specific brain regions such as the subgranular zone of the dentate gyrus and subventricular zone of the lateral ventricle. Adult neurogenesis is vital neural plasticity, providing a potential mechanism for the brain to adapt and reorganize in response to environmental cues and experiences. Cutting-edge research and sophisticated imaging techniques, such as two-photon microscopy, MRI, optogenetic, and stem-cell-based therapies have provided deeper insight into the study of adult neurogenesis.
Conclusion: The study of neurogenesis is important for understanding nervous system development, physiology, pathology, and exploring neuroplasticity. Its advancement is challenged by some ethical concerns regarding embryonic, pluripotent stem cells, and the need for safe, and noninvasive study methods. Although recent breakthroughs in neuroimaging, microscopic techniques, and genetic tools are aiding real-time study of adult neurogenesis.
Adult neurogenesis is the generation of new neurons coordinated by complex systems, involving neuroplasticity and persisting throughout life [1], these neurons originate from neural stem cells [2]. Altman’s groundbreaking research with the thymidine autoradiograph technique which labels dividing cells with [3H]-thymidine, combined into the replicating DNA provided the first proof of the generation of new neurons in adult rats [3]. Positive evidence of neurogenesis in the hippocampus was later reported in 1998, following a study on the post-mortem tissue from cancer patients who had received therapeutic injections of Bromodeoxyuridine [4]. Events of adult neurogenesis occur in different brain regions such as the subventricular zone of the lateral ventricles and subgranular zone of the dentate gyrus and involve complex regulatory pathways, the processes are facilitated by modulators that play a critical role in its occurrence consisting, of signaling transduction pathways, metabolic factors, the vascular and immune systems, and epigenetic regulation. Adult neurogenesis is adversely impacted by neurodegeneration due to the various modulators [5]. The repair of damaged tissue and enhancement of neurogenesis is now possible through some advanced processes like stem cell-based therapies. Furthermore, understanding the concept of neurogenesis via in vivo study has been possible through advanced imaging techniques such as nuclear magnetic resonance spectroscopy, positron emission tomography, and next-generation sequencing or single-cell sequencing [6]. These procedures require meticulous supervision, regulation, substantial energy costs, and certain risks [7].
Molecular signaling pathway in adult neurogenesis
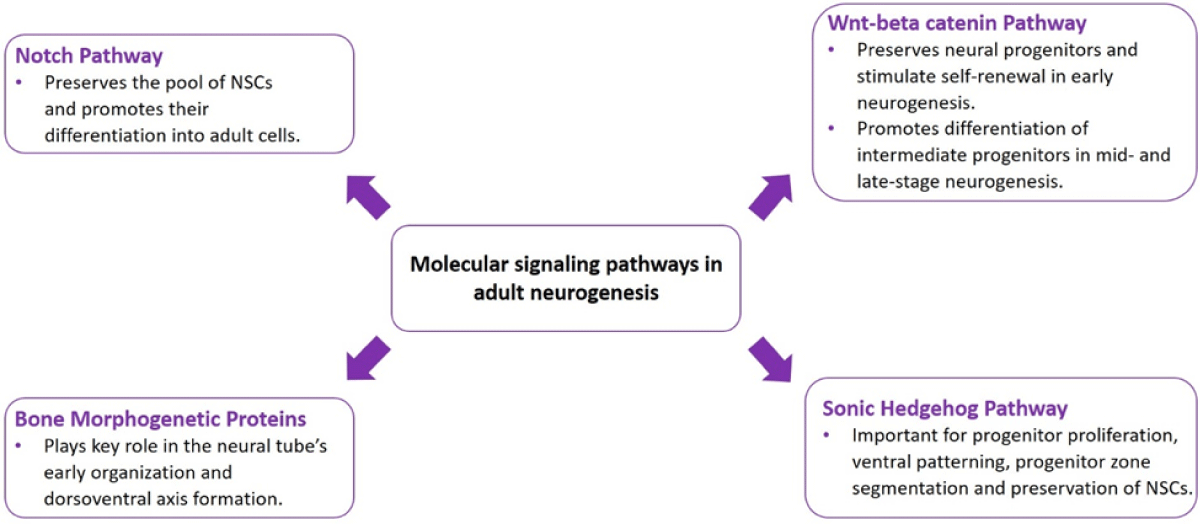
Specific pathways regulate self-renewal, proliferation, differentiation of neuronal stem cells, migration, and integration of developing neurons’ functions in the adult brain [8]. These pathways do not occur independently, as they are regulated by extrinsic environmental influences and intrinsic genetic factors. Adverse environmental situations, for instance, may set off acute stress reactions, which in turn may cause the adrenal cortex to release glucocorticoids. Targeting vital receptors in this signaling cascade, this hormone can penetrate the blood-brain barrier, leading to transactivation or trans-repression of specific genes of some signaling pathways like Forkhead box protein O3 pathway, Transforming growth factor Beta pathway, and Hedgehog pathway [9] (Figure 1).
Figure 1: Schematic diagram showing the molecular signaling pathways in adult neurogenesis.
Notch signaling pathway
The notch pathway regulates various processes, such as cell proliferation, differentiation, and apoptosis. There is increasing proof that this route has separate functions in the sub-ventricular and sub-granular zones of the adult brain in terms of sustaining and developing neural stem cells. It preserves the pool of neural stem cells and promotes their differentiation into adult cells by means of downstream transcriptional effectors and Notch ligands (Delta, LAG-2, APX-) receptors [10].
Wnt-beta catenin pathway
Wnt ligands are a family of secreted glycoproteins involved in a large variety of central nervous system developmental and adult processes. Lineage tracing experiments observed the existence of Wnt/b-catenin-responsive stem cells in both the SVZ and SGZ niches [11]. Wnt signaling stimulates cortical neural progenitor cells to self-renew and differentiate according to developmental phases [12,13]. In mid- and late-stage neurogenesis, the pathway promotes the differentiation of intermediate progenitors; in early neurogenesis, however, it preserves neural progenitors and stimulates self-renewal [14-16].
Bone Morphogenetic Proteins (BMPs)
They are growth factors under the Transforming growth factor beta superfamily. The effects of BMP signaling on the development of the nervous system vary, and the particular cellular response that BMPs elicit is dependent on the properties of the target cell and the signaling milieu. The neural tube’s early organization and dorsoventral axis formation depend on the precise interaction between BMPs and their natural antagonist, Noggin, Chordin, and Follistatin. BMPs also continue to control a wide range of cellular activities later on in embryogenesis, such as proliferation, apoptosis, neurogenesis, and gliogenesis [17,18].
Sonic Hedgehog pathway
During embryonic development, the multifunctional signaling protein known as Sonic Hedgehog (Shh) Shh is needed for progenitor proliferation, ventral patterning, progenitor zone segmentation, and preservation of NSCs in the SGZ and SVZ throughout life in forebrain development [19].
Adult neurogenesis in drosophila, rodents and humans
Adult neurogenesis in drosophila: The brains of drosophila and other invertebrates were initially considered to be hardwired and incapable of neurogenesis. This was supported by the fact that neuroblasts, which are precursors to new neurons were eliminated before the adult fly ecloses, so it was unclear how new neurons could be generated [20]. However, recent studies have shown the presence of proliferating cells in adult drosophila brains. It has been found to occur at the vital period during day 1 to 6 post eclosion, particularly in the antennal lobes, central brain, sub-aesophageal ganglion, and medulla cortex of the optic lobe [21-23]. Neuronal activity, genetic modification, and damage have all been demonstrated to initiate neurogenesis in drosophila [24]. Adult neurogenesis is considered a homeostatic mechanism that helps to maintain the number of cells throughout adult life [24].
Adult neurogenesis in mammalians (Rodents and Humans)
Neurogenesis in the subgranular zone of dentate gyrus: The subgranular zone, which is situated between the hilus and granular layer, contains neural precursor cells that give rise to new granular neurons via the following cascade: Quiescent neuroprogenitor cells proliferate and give rise to amplifying neuroprogenitors, a transient population that in turn proliferate into neuronal committed neuroblasts. The neuroblasts mature into granular neurons that are integrated into the neural circuit. Newly formed neurons project their dendrites towards the molecular layer, their cellular processes towards the hilum, and their mossy fibers into other areas of the hippocampus. These neurons play roles in processing information and facilitating temporal separation of spatial memories [25,26].
Neurogenesis in the subventricular zone of the lateral ventricle: The subventricular zone, found on the lateral wall of the lateral ventricles, contains specialized astrocytes known as B cells, the primary source for generating new neurons. The B cells proliferate and give rise to C cells, the transient amplifying population of the system. C cells in turn give rise to neuroblasts or A cells. The A cell migrates to the olfactory bulb through the Rostral Migratory Stream (RMS), where it differentiates into periglomerular interneurons and granule cells [27]. These neurons are involved in olfactory memory formation and odorant discrimination [28]. The neuroblasts from the SVZ may also migrate to the basal ganglia and cerebral cortex [29].
Methodologies for investigating adult neurogenesis
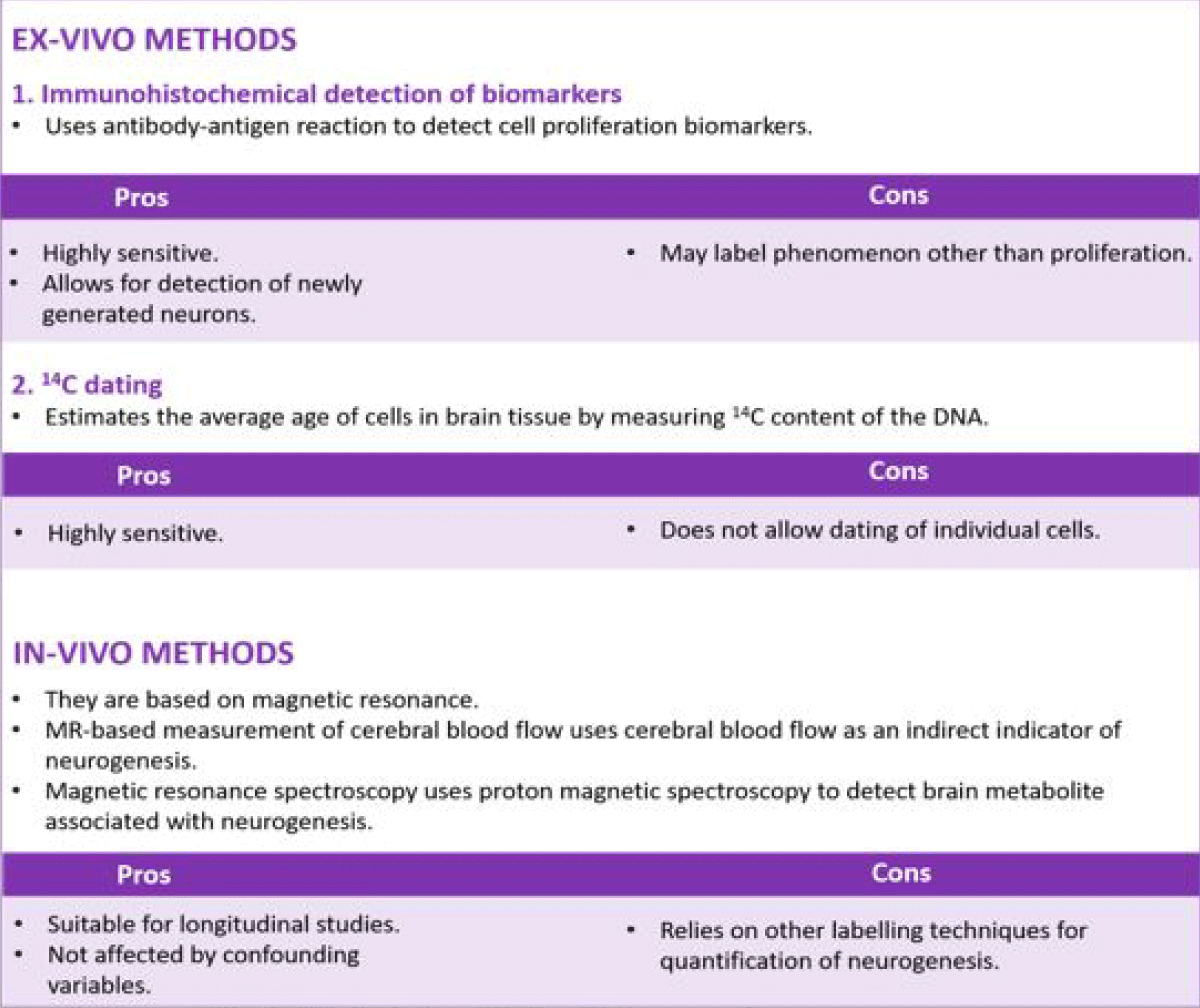
Ex-vivo methods: They are performed directly on brain tissue which is extracted from experimental animals post-mortem or from humans through biopsy. These methods do not support longitudinal studies, and obtained results could be influenced by confounding factors such as cause of death, presence of illness, age of death, and post-mortem interval [30].
Immunohistochemical detection of biomarkers: The use of immunohistochemistry (IHC) to study neurogenesis relies on the expression of cell proliferation biomarkers or cell-specific biomarkers. Appropriate antibodies specific to the tissue are used to label these biomarkers, making it possible to visualize and study proliferating cells. Some proliferation biomarkers include: Ki-67; MCM2, minichromosome maintenance protein; and PCNA, proliferating cell nuclear antigen. Common cell-specific biomarkers include but are not limited to DCX, doublecortin; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; and nestin. A distinct IHC-based technique is the Bromodeoxyuridine (BrdU) assay, in which initial administration of BrdU is followed up with the application of anti-BrdU antibody to identify proliferating cells. The administered BrdU is incorporated into the DNA during the S phase of the cell cycle and it is transmitted to daughter cells, provided it is not diluted from many rounds of proliferation [31]. BrdU labeling is highly sensitive to detecting proliferating cells in comparison to other IHC methods, and it allows for the detection of newly generated granule [32]. However, the availability of BrdU in the brain can be affected by the conditions of the BBB (e.g. inflammation, irradiation, and trauma). Also, BrdU may label phenomena other than proliferation such as DNA turnover or repair or abortive reentry in the cell cycle during apoptosis [32].
14C dating: The technique estimates the average age of cells in brain tissue by measuring the 14C content of the DNA. 14C dating has been used to demonstrate that adult neurogenesis in the human neocortex is very limited or absent, although gliogenesis might occur [33]. This technique is highly sensitive as it can detect newborn cells down to 1% of the population. However, it can only yield the average age of cells in the tissue and does not allow the dating of individual cells.
In-vivo methods: The study of neurogenesis with in-vivo methods relies heavily on Magnetic Resonance Imaging (MRI). Hence, they utilize MRI scanners which are widely available in hospitals and research centers. MR-based in-vivo methods can be performed on live individuals, thus supporting longitudinal studies. In addition, confounding variables associated with ex-vivo methods such as cause or age of death need not be taken into consideration. However, MR methods rely on correlation with other labeling techniques for quantification of neurogenesis, they also require extensive validation in humans and rodents to ensure they are specific for neurogenesis.
MR-based measurement of Cerebral Blood Flow (CBV)
The use of CBV as an indicator for adult neurogenesis is based on the theory that increased CBV is correlated with angiogenesis, which is in turn correlated with neurogenesis [33]. Angiogenesis has been shown to take place in the neurogenic zone of the hippocampus. Also, angiogenesis and neurogenesis are elevated in the hippocampus following physical exercise [34]. The indirect link between CBV and neurogenesis was demonstrated by Pereira and colleagues who were able to show increased CBV in the dentate gyrus after a prolonged period of exercise. Elevated CBV was found to be correlated with an increased number of 1- to 3-week-old BrdU+ cells, which validates the occurrence of neurogenesis. Cerebral blood volume is measured by using gadolinium chelate as a contrast agent in combination with MRI [35]. The contrast agent is administered systemically, and it is restricted to the intravascular space by the blood-brain barrier due to its low lipophilicity. The contrast agent possesses paramagnetic properties, which causes it to produce a decrease in T1 signal intensity. Alterations in the emitted signal are used to generate a map of cerebral blood volume.
Magnetic resonance spectroscopy
Herein, proton magnetic spectroscopy (1H-MRS) is used to detect a brain metabolite associated with neurogenesis. Following exposure to a magnetic field, the protons of this metabolite emit signals after irradiation with radiofrequency waves. The emitted signal, which is known as Free Induction Decay (FID), is converted to a graph of peaks (spectrum) via a mathematical process known as Fourier transform [36]. The area under these peaks represents the proton content of different metabolites. This method was initially utilized by Manganas and colleagues to detect a metabolite localized in neural progenitor cells (NPCs) of rodents [37]. The association of the metabolite with neurogenesis was confirmed with micro MR spectroscopy, which detected the metabolite in the hippocampus and cortex after NPC transplantation [37]. In addition, the amount of metabolite detected correlated with the number of BrdU+ cells in the hippocampus after electroconvulsive shock-induced increase in endogenous neurogenesis. The use of this method to study neurogenesis in humans is currently limited owing to concerns about its reliability [35] (Figure 2).
Figure 2: Summary of methodologies for investigating adult neurogenesis.
Potential function of adult neurogenesis
Memory and learning: The discovery of ongoing neurogenesis in the adult brain, particularly in the hippocampus, challenged long-held beliefs about the brain’s limited regenerative capacity [38]. This phenomenon, initially not fully regarded, has since been widely accepted, with implications reaching far beyond basic neuroscience. Adult neurogenesis represents a form of neural plasticity, providing a potential mechanism for the brain to adapt and reorganize in response to environmental cues and experiences [39]. Advanced imaging techniques, such as two-photon microscopy, have brought about a better understanding of adult neurogenesis by allowing for the visualization of adult-born neuron integration into existing circuits [40]. Studies have shown that these newly generated neurons exhibit unique properties, including enhanced synaptic plasticity and excitability, which are believed to play an important role in memory formation [41,42]. The integration of adult-born neurons into the hippocampal circuitry is thought to contribute to the encoding and consolidation of memories, highlighting the functional significance of adult neurogenesis in cognitive processes [43]. Beyond memory formation, adult neurogenesis has also been significantly correlated to cognitive flexibility [44], which is the brain’s ability to adapt behavior in response to changing environments. Additionally, pharmacological interventions aimed at enhancing neurogenesis have shown promise in preclinical studies as potential treatments for neurodegenerative diseases [45,46]. For example, the administration of growth factors or small molecules that promote neurogenesis has been shown to improve cognitive function and reduce neuronal loss in animal models of Alzheimer’s and Parkinson’s [47,48]. From findings, strategies known to promote neurogenesis, such as physical exercise, can significantly enhance cognitive function and memory in both animal models [49] and in humans [50]. Exercise has been found to stimulate the production of new neurons in the hippocampus [51,52], a brain region responsible for learning and memory, suggesting that promoting neurogenesis could be a new approach to combating cognitive decline associated with neurodegenerative diseases. These findings highlight the therapeutic potential of targeting neurogenesis as a new approach to treating neurodegenerative disorders and the treatment of depression and anxiety as previously recommended [53]. The formation of new therapies that specifically target neurogenesis could potentially provide more effective and better-tolerated treatments for these debilitating disorders.
Mood regulation: While much of the focus on adult neurogenesis research has been on its role in neurodegenerative diseases such as Alzheimer’s and Parkinson’s, there is growing evidence to suggest that adult neurogenesis may also impact the progression of psychiatric disorders, particularly depression and anxiety [54,55]. Adult neurogenesis, the process by which new neurons are generated in the adult brain, is a significant player in mood regulation and emotional behavior. Noteworthy, as previously mentioned, is that the hippocampus, involved in learning, memory, and emotional regulation, is one of the primary sites of adult neurogenesis. Several past studies have indicated that a decrease in neurogenesis in the hippocampus may be linked to the pathophysiology of depression and anxiety [56,57]. Postmortem studies of depressed individuals have shown reduced hippocampal neurogenesis [58] and animal studies have demonstrated that stress, a major risk factor for depression and anxiety, can suppress neurogenesis in the hippocampus [59].
Neuronal plasticity and repair: Neuronal plasticity, the brain’s ability to reorganize and adapt in response to new experiences, is a fundamental process that underlies learning, memory, and recovery from injury [60]. Adult neurogenesis plays an important role in neuronal plasticity and repair by integrating these new neurons into existing circuits. The integration of adult-born neurons into existing circuits is thought to contribute to neuronal plasticity by providing a source of new cells that can form connections with other neurons [60]. This process, known as synaptic integration, allows for the formation of new synaptic connections and the strengthening of existing ones, which is essential for learning and memory. Moreover, adult neurogenesis may also play a role in neuronal repair following injury or degeneration. In conditions such as neurodegenerative diseases or brain injury, the loss of neurons can lead to cognitive decline and functional impairments. The generation of new neurons through adult neurogenesis may help to replace lost or damaged neurons, potentially restoring cognitive function and neuronal integrity [62]. Recent findings in the field of adult neurogenesis have identified collectively its role in mood regulation, neuronal plasticity, and repair in the adult brain [61]. Among these, the major advancement is the identification of specific molecular and cellular mechanisms that regulate adult neurogenesis and its impact on behavior. Studies have identified key molecular pathways involved in adult neurogenesis, such as the Notch signaling pathway, which plays an important role in the maintenance and differentiation of neural stem cells [62]. The disruption of notch signaling has been shown to impair adult neurogenesis and lead to mood-related behaviors in animal models [63], highlighting its importance in mood regulation. Furthermore, recent research has revealed the role of microRNAs (miRNAs) in regulating adult neurogenesis. MiRNAs are small RNA molecules that can modulate gene expression and have been implicated in various aspects of neurogenesis, including cell proliferation, differentiation, and survival [64]. Dysregulation of miRNAs has been linked to mood disorders and neurodegenerative diseases, suggesting that targeting miRNAs could be a potential therapeutic strategy for these conditions [65]. In addition to molecular mechanisms, recent studies have also focused on understanding the functional implications of adult neurogenesis. Using advanced imaging techniques has shown that adult-born neurons integrate into existing circuits and contribute to specific brain functions, such as pattern separation and memory formation [66]. These findings provide further evidence for the role of adult neurogenesis in cognitive processes and highlight its potential as a target for enhancing cognitive function in health and disease.
Environmental enrichment: The potential of neurogenesis, particularly in the context of environmental enrichment, opens up new prospects for brain health and disease prevention. Recent studies have shown that lifestyle interventions, such as physical activity, social interaction, and cognitive stimulation, can enhance adult neurogenesis, which in turn may promote brain health and resilience against neurological disorders [67,68]. One promising area of research is the potential of neurogenesis to improve cognitive function and memory. Studies have demonstrated that environmental enrichment can enhance learning and memory in animal models, and this effect is thought to be mediated, at least in part, by increased adult neurogenesis [69,70]. This suggests that lifestyle interventions aimed at promoting neurogenesis could have cognitive benefits, particularly in aging populations or those at risk of cognitive decline.
Furthermore, the potential of neurogenesis extends to its role in mood regulation and mental health as described earlier. Studies have shown that environmental factors, such as stress and depression, can negatively impact adult neurogenesis [71], while interventions that promote neurogenesis, such as antidepressant treatment or environmental enrichment, can improve mood-related behaviors [72]. This highlights the potential of neurogenesis as a target for interventions aimed at improving mental health and well-being. In addition to its role in cognitive function and mood regulation, the potential of neurogenesis in brain repair and neuroprotection is also of great interest. Studies have shown that adult-born neurons can integrate into existing circuits and contribute to functional recovery following brain injury or disease [73]. This suggests that promoting neurogenesis through lifestyle interventions could enhance the brain’s ability to repair itself and protect against neurodegenerative diseases.
Advances in adult neurogenesis
Technological advances: Recent technological advances have been seen in our understanding of adult neurogenesis. One major innovation is the development of advanced imaging techniques, particularly two-photon microscopy. This technology allows researchers to observe neurogenesis in real-time within living animals, providing a better understanding of the process [74]. The tracking of individual cells over time has yielded easy study on the proliferation, migration, and integration of new neurons into existing neural circuits. Another significant advancement is the use of genetic tools, such as optogenetics, to selectively manipulate the activity of newly generated neurons. Optogenetics involves genetically modifying neurons to express light-sensitive proteins, allowing for the control of activity with precise light stimulation [75]. The easy activation and inhibition of these neurons at specific times provides a better understanding of the functional roles of new neurons in various brain functions, such as learning, memory, and mood regulation. Furthermore, advancements in molecular biology techniques have enabled researchers to label and track specific populations of new neurons. Through the use of genetic markers or viral vectors, newborn neurons are labeled, and development and integration are traced into neural circuits over time [76]. This approach is a development that provides answers to the factors that regulate neurogenesis, such as environmental stimuli, hormonal signals, and neurotransmitters.
Current research: The discovery of adult neurogenesis, the mechanism by which new neurons are formed in the adult brain, has brought about new therapeutic interventions in various neurological conditions. Neurobiology researchers are actively exploring ways to harness this process to enhance brain function and promote recovery from injuries and diseases. One promising avenue of research is the use of stem cell-based therapies to enhance neurogenesis and repair damaged brain tissue. Stem cells have the ability to differentiate into various cell types, including neurons and their transplantation into the brain, helping to restore lost neurons and improve neurological function [77]. Animal models in preclinical studies have shown promising results, demonstrating the potential of stem cell-based therapies to promote recovery after brain injury or stroke [78]. These therapies hold great potential for restoring lost function and improving the quality of life for patients with neurological disorders. In addition to stem cell-based therapies, researchers are also investigating drugs that can promote neurogenesis as potential treatments for psychiatric and neurological disorders. One class of drugs that has shown promise in this regard is antidepressants, such as Selective Serotonin Reuptake Inhibitors (SSRIs) (e.g. fluoxetine (Prozac), sertraline (Zoloft), and escitalopram (Lexapro), and Tricyclic Antidepressants (TCAs) (e.g. amitriptyline (Elavil) and imipramine (Tofranil) have been studied for their potential to promote neurogenesis [79]. These antidepressants can stimulate neurogenesis in the adult brain, particularly in the hippocampus. Antidepressants may help to alleviate symptoms of depression and other mood disorders by promoting neurogenesis. Research in animal models suggests that enhancing neurogenesis can improve mood and cognitive function, providing a “renewed hope” for therapeutic intervention in these debilitating conditions.
Ethical concerns, challenges and future direction
Research in adult neurogenesis faces several ethical issues and challenges. Adult stem cell research, particularly neural stem cells, provides crucial insights into the mechanisms of neurogenesis and is vital for developing therapies aimed at enhancing neurogenesis to treat and manage neurodegenerative diseases. However, the long-term safety and efficacy of stem cell therapies remain uncertain, and procuring stem cells, especially embryonic stem cells, genetic manipulation techniques, which allow scientists to modify stem cells, poses ethical challenges in research. Among these concerns is the potential for side effects stemming from the manipulation of stem cells, and the alteration of other aspects of brain function or behavior. The use of advanced imaging techniques like MR-based measurement of cerebral blood volume (CBV) and magnetic spectroscopy, while non-invasive, also introduces concerns about the extent of permissible human experimentation, the management of incidental findings such as tumors or malformations, and the application of research findings, especially in terms of developing interventions or treatments. Hence, key contributions to research in adult neurogenesis must navigate these challenges. Future directions should include the development of research policies that prioritize ethical considerations, promote transparency, and ensure equitable access to stem cell therapies Additionally, improving the non-invasive nature and accuracy of imaging techniques and the safety of genetic manipulation is also crucial. Overall, a multidisciplinary approach that integrates scientific, ethical, and regulatory policies is essential for advancing adult neurogenesis research.
Neurogenesis is critical in the understanding of nervous system development, physiology, and pathology and also plays a vital role in exploring the concept of neuroplasticity. Amidst this function, ethical issues still pose such as the use of embryonic and pluripotent stem cells in its advancement and translation in human studies. Investigations on neurogenesis in living humans have also been challenged by the lack of safe and noninvasive methods of study. Recent neuroimaging, microscopic techniques, and genetic tools have been developed to help biologists study adult neurogenesis in real-time.
Authors contribution
Conceptualization and Design: Gideon S.A., Tolulope J.G., Anene-Ogbe C.M., Olanrewaju O.O., Writing of Manuscript: Gideon S.A., Joy W.E. Olanrewaju O.O., Tolulope J.G., Anene-Ogbe C.M., Glory F., Joshua A.Y., All authors reviewed the manuscript.
- Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M, Rábano A, Llorens-Martín M. Evidences for adult hippocampal neurogenesis in humans. J Neurosci. 2021;41(12):2541-2553. Available from: https://doi.org/10.1523/jneurosci.0675-20.2020
- Denoth-Lippuner A, Jessberger S. Formation and integration of new neurons in the adult hippocampus. Nat Rev Neurosci. 2021;22(4):223-236. Available from: https://doi.org/10.1038/s41583-021-00433-z
- Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016;1644:127-140. Available from: https://doi.org/10.1016/j.brainres.2016.05.015
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313-1317. Available from: https://doi.org/10.1038/3305
- Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: a systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):93-112. Available from: https://doi.org/10.1002/ajmg.b.32429
- Kuhn HG, Toda T, Gage FH. Adult hippocampal neurogenesis: a coming-of-age story. J Neurosci. 2018;38(49):10401-10410. Available from: https://doi.org/10.1523/jneurosci.2144-18.2018
- Kempermann G. Adult neurogenesis: an evolutionary perspective. Cold Spring Harb Perspect Biol. 2016;8(2). Available from: https://doi.org/10.1101/cshperspect.a018986
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310(5748):679-683. Available from: https://doi.org/10.1126/science.1115360
- Egeland M, Zunszain PA, Pariante CM. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat Rev Neurosci. 2015;16(4):189-200. Available from: https://doi.org/10.1038/nrn3855
- Choe Y, Pleasure SJ, Mira H. Control of adult neurogenesis by short-range morphogenic-signaling molecules. Cold Spring Harb Perspect Biol. 2016;8(3):a018887. Available from: https://doi.org/10.1101/cshperspect.a018887
- Bowman AN, Van Amerongen R, Palmer TD, Nusse R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin–responsive neural stem cells. Proc Natl Acad Sci U S A. 2013;110(18):7324-7329. Available from: https://doi.org/10.1073/pnas.1305411110
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131(12):2791-801. Available from: https://doi.org/10.1242/dev.01165
- Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J Neurosci. 2011;31(5):1676-1687. Available from: https://doi.org/10.1523/jneurosci.5404-10.2011
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long-term potentiation. J Biol Chem. 2006;281(17):11910-11916. Available from: https://doi.org/10.1074/jbc.m511920200
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311(1):223-237. Available from: https://doi.org/10.1016/j.ydbio.2007.08.038
- Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized β-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol. 2007;309(2):285-297. Available from: https://doi.org/10.1016/j.ydbio.2007.07.013
- Panchision DM, McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12(4):478-487. Available from: https://doi.org/10.1016/s0959-437x(02)00329-5
- Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11(7):493-501. Available from: https://doi.org/10.1038/nrc3079
- Li G, Hidalgo A. Adult neurogenesis in the Drosophila brain: the evidence and the void. Int J Mol Sci. 2020;21(18):6653. Available from: https://doi.org/10.3390/ijms21186653
- Fernández-Hernández I, Rhiner C, Moreno E. Adult neurogenesis in Drosophila. Cell Rep. 2013;3(6):1857-1865. Available from: https://doi.org/10.1016/j.celrep.2013.05.034
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149(1):134-148. Available from: https://doi.org/10.1016/0012-1606(92)90270-q
- Kato K, Awasaki T, Ito K. Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development. 2009;136(1):51-59. Available from: https://doi.org/10.1242/dev.023366
- Li G, Forero MG, Wentzell JS, Durmus I, Wolf R, Anthoney NC, et al. A Toll-receptor map underlies structural brain plasticity. Elife. 2020;9. Available from: https://doi.org/10.7554/elife.52743
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598-602. Available from: https://doi.org/10.1126/science.1248903
- Jacobs BL, Van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5(3):262-269. Available from: https://doi.org/10.1038/sj.mp.4000712
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978-981. Available from: https://doi.org/10.1126/science.271.5251.978
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687-702. Available from: https://doi.org/10.1016/j.neuron.2011.05.001
- Abdissa D, Hamba N, Gerbi A. Review article on adult neurogenesis in humans. Transl Res Anat. 2020;20:100074. Available from: https://doi.org/10.1016/j.tria.2020.100074
- Sierra A, Encinas JM, Maletic-Savatic M. Adult human neurogenesis: from microscopy to magnetic resonance imaging. Front Neurosci. 2011;5:47. Available from: https://doi.org/10.3389/fnins.2011.00047
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564-12568. Available from: https://doi.org/10.1073/pnas.0605177103
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133-143. Available from: https://doi.org/10.1016/j.cell.2005.04.028
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680-8685. Available from: https://doi.org/10.1523/jneurosci.1731-05.2005
- Novotny E, Ashwal S, Shevell M. Proton magnetic resonance spectroscopy: an emerging technology in pediatric neurology research. Pediatr Res. 1998;44(1):1-10. Available from: https://doi.org/10.1203/00006450-199807000-00001
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980-985. Available from: https://doi.org/10.1126/science.1147851
- Riddle DR, Lichtenwalner RJ. Neurogenesis in the adult and aging brain. Brain Aging. 2007:127-158. Available from: https://www.ncbi.nlm.nih.gov/books/NBK3874/
- Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24(1):67-87. Available from: https://doi.org/10.1038/s41380-018-0036-2
- Mizuta K, Sato M. Multiphoton imaging of hippocampal neural circuits: techniques and biological insights into region-, cell-type-, and pathway-specific functions. Neurophotonics. 2024;11(3):033406. Available from: https://doi.org/10.1117/1.nph.11.3.033406
- Chen L, Cummings KA, Mau W, Zaki Y, Dong Z, Rabinowitz S, et al. The role of intrinsic excitability in the evolution of memory: Significance in memory allocation, consolidation, and updating. Neurobiol Learn Mem. 2020;173:107266. Available from: https://doi.org/10.1016/j.nlm.2020.107266
- Abrous DN, Wojtowicz JM. Interaction between neurogenesis and hippocampal memory system: new vistas. Cold Spring Harb Perspect Biol. 2015;7(6). Available from: https://doi.org/10.1101/cshperspect.a018952
- Yau SY, Li A, So KF. Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast. 2015;2015:717958. Available from: https://doi.org/10.1155/2015/717958
- Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat Rev Neurosci. 2017;18(6):335-346. Available from: https://doi.org/10.1038/nrn.2017.45
- Palanisamy CP, Pei J, Alugoju P, Anthikapalli NV, Jayaraman S, Veeraraghavan VP, et al. New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics. 2023;13(12):4138-4165. Available from: https://doi.org/10.7150/thno.83066
- Culig L, Chu X, Bohr VA. Neurogenesis in aging and age-related neurodegenerative diseases. Ageing Res Rev. 2022;78:101636. Available from: https://doi.org/10.1016/j.arr.2022.101636
- Kim MY, Kim MJ, Lee C, Lee J, Kim SS, Hong S, et al. Trametinib activates endogenous neurogenesis and recovers neuropathology in a model of Alzheimer’s disease. Exp Mol Med. 2023;55(10):2177-2189. Available from: https://doi.org/10.1038/s12276-023-01073-2
- Jastrzębski MK, Wójcik P, Stępnicki P, Kaczor AA. Effects of small molecules on neurogenesis: neuronal proliferation and differentiation. Acta Pharm Sin B. 2023 Oct 20. [Epub ahead of print]. Available from: https://doi.org/10.1016/j.apsb.2023.10.015
- Liu Y, Chu JM, Yan T, Zhang Y, Chen Y, Chang RC, et al. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice. J Neuroinflammation. 2020;17:4. Available from: https://doi.org/10.1186/s12974-019-1653-7
- Özbeyli D, Sarı G, Özkan N, Karademir B, Yüksel M, Kaya ÖT, et al. Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav Brain Res. 2017;328:159-77. Available from: https://doi.org/10.1016/j.bbr.2017.03.044
- Martini F, Régis Leite M, Gonçalves Rosa S, Pregardier Klann I, Wayne Nogueira C. Strength exercise suppresses STZ-induced spatial memory impairment and modulates BDNF/ERK-CAMKII/CREB signaling pathway in the hippocampus of mice. Cell Biochem Funct. 2020;38(2):213-221. Available from: https://doi.org/10.1002/cbf.3470
- Farzi MA, Sadigh-Eteghad S, Ebrahimi K, Talebi M. Exercise improves recognition memory and acetylcholinesterase activity in the beta amyloid-induced rat model of Alzheimer’s disease. Ann Neurosci. 2019;25(3):121-125. Available from: https://doi.org/10.1159/000488580
- Bonanni R, Cariati I, Tarantino U, D’Arcangelo G, Tancredi V. Physical exercise and health: a focus on its protective role in neurodegenerative diseases. J Funct Morphol Kinesiol. 2022;7(2):38. Available from: https://doi.org/10.3390/jfmk7020038
- Latchney SE, Eisch AJ. Therapeutic application of neural stem cells and adult neurogenesis for neurodegenerative disorders: regeneration and beyond. Eur J Neurodegener Dis. 2012;1(3):335-51. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4340249/
- Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8(9). Available from: https://doi.org/10.1101/cshperspect.a019026
- Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270-276. Available from: https://doi.org/10.1016/j.brainres.2016.01.023
- Alonso M, Petit AC, Lledo PM. The impact of adult neurogenesis on affective functions: of mice and men. Mol Psychiatry. 2024;29(8):2527-2542. Available from: https://doi.org/10.1038/s41380-024-02504-w
- Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417-426. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC524959/
- Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention, and treatment. Sig Transduct Target Ther. 2024;9:30. Available from: https://doi.org/10.1038/s41392-024-01738-y
- Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model—a comprehensive review. Biomed Pharmacother. 2018;105:1205-1222. Available from: https://doi.org/10.1016/j.biopha.2018.05.086
- Petković A, Chaudhury D. Encore: behavioural animal models of stress, depression, and mood disorders. Front Behav Neurosci. 2022;16:931964. Available from: https://doi.org/10.3389/fnbeh.2022.931964
- Dieni CV, Gonzalez JC, Overstreet-Wadiche L. Multifaceted circuit functions of adult-born neurons. F1000Res. 2019;8. Available from: https://doi.org/10.12688/f1000research.20642.1
- Ghosh HS. Adult neurogenesis and the promise of adult neural stem cells. J Exp Neurosci. 2019;13:1179069519856876. Available from: https://doi.org/10.1177/1179069519856876
- Jones KL, Zhou M, Jhaveri DJ. Dissecting the role of adult hippocampal neurogenesis towards resilience versus susceptibility to stress-related mood disorders. npj Sci Learn. 2022;7(1):16. Available from: https://doi.org/10.1038/s41539-022-00133-y
- Lampada A, Taylor V. Notch signaling as a master regulator of adult neurogenesis. Front Neurosci. 2023 Jun 29;17:1179011. Available from: https://doi.org/10.3389/fnins.2023.1179011
- Hussain G, Akram R, Anwar H, Sajid F, Iman T, Han HS, et al. Adult neurogenesis: a real hope or a delusion?. Neural Regen Res. 2024;19(1):6-15. Available from: https://doi.org/10.4103/1673-5374.375317
- Tsujimura K, Shiohama T, Takahashi E. microRNA biology on brain development and neuroimaging approach. Brain Sci. 2022;12(10):1366. Available from: https://doi.org/10.3390/brainsci12101366
- Mohammed OA, Elballal MS, El-Husseiny AA, Khidr EG, El Tabaa MM, Elazazy O, et al. Unraveling the role of miRNAs in the diagnosis, progression, and therapeutic intervention of Parkinson’s disease. Pathol Res Pract. 2023:155023. Available from: https://doi.org/10.1016/j.prp.2023.155023
- Kim TA, Syty MD, Wu K, Ge S. Adult hippocampal neurogenesis and its impairment in Alzheimer’s disease. Zool Res. 2022 May 5;43(3):481-96. Available from: https://doi.org/10.24272/j.issn.2095-8137.2021.479
- Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017;2017(1):3589271. Available from: https://doi.org/10.1155/2017/3589271
- Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. 2018;9:509. Available from: https://doi.org/10.3389/fpsyg.2018.00509
- Hullinger R, O’Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126-34. Available from: https://doi.org/10.1016/j.nlm.2015.08.006
- Zentall TR. Effect of environmental enrichment on the brain and on learning and cognition by animals. Animals. 2021 Mar 31;11(4):973. Available from: https://doi.org/10.3390/ani11040973
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12-21. Available from: https://doi.org/10.1016/j.expneurol.2011.01.008
- Ramírez-Rodríguez GB, Vega-Rivera NM, Meneses-San Juan D, Ortiz-López L, Estrada-Camarena EM, Flores-Ramos M. Short daily exposure to environmental enrichment, fluoxetine, or their combination reverses deterioration of the coat and anhedonia behaviors with differential effects on hippocampal neurogenesis in chronically stressed mice. Int J Mol Sci. 2021;22(20):10976. Available from: https://doi.org/10.3390/ijms222010976
- Llorente V, Velarde P, Desco M, Gómez-Gaviro MV. Current understanding of the neural stem cell niches. Cells. 2022;11(19):3002. Available from: https://doi.org/10.3390/cells11193002
- Liu B, Li Y, Ren M, Li X. Targeted approaches to delineate neuronal morphology during early development. Front Cell Neurosci. 2023;17:1259360. Available from: https://doi.org/10.3389/fncel.2023.1259360
- Hassan AU, Hassan G, Rasool Z. Role of stem cells in treatment of neurological disorder. Int J Health Sci. 2009 Jul;3(2):227-33. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3068820/
- Pinosanu LR, Wolff N, Olaru DG, Popa-Wagner A. Stem cell treatments in preclinical relevant stroke models. Curr Health Sci J. 2023;49(4):487-494. Available from: https://doi.org/10.12865/chsj.49.04.02
- Nalamolu KR, Chelluboina B, Fornal CA, Challa SR, Pinson DM, Wang DZ, et al. Stem cell treatment improves post stroke neurological outcomes: a comparative study in male and female rats. Stroke Vasc Neurol. 2021;6(4):519-527. Available from: https://doi.org/10.1136/svn-2020-000834
- Sadanandan N, Saft M, Gonzales-Portillo B, Borlongan CV. Multipronged attack of stem cell therapy in treating the neurological and neuropsychiatric symptoms of epilepsy. Front Pharmacol. 2021;12:596287. Available from: https://doi.org/10.3389/fphar.2021.596287
- de Oliveira CL, Bolzan JA, Surget A, Belzung C. Do antidepressants promote neurogenesis in adult hippocampus? A systematic review and meta-analysis on naive rodents. Pharmacol Ther. 2020;210:107515. Available from: https://doi.org/10.1016/j.pharmthera.2020.107515