More Information
Submitted: September 10, 2024 | Approved: September 17, 2024 | Published: September 18, 2024
How to cite this article: Makar TK, Bryant J, Shim B, Keledjian K, Davis H, Ghosh M, et al. Neuroprotective Effect of 7,8-dihydroxyflavone in a Mouse Model of HIV-Associated Neurocognitive Disorder (HAND). J Neurosci Neurol Disord. 2024; 8(2): 090-105. Available from: https://dx.doi.org/10.29328/journal.jnnd.1001101.
DOI: 10.29328/journal.jnnd.1001101
Copyright License: © 2024 Makar TK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: HAND; BDNF; TrkB; DHF; Transgenic; Transgenic mice-Tg26; Neurodegeneration; Neuroprotection; Antioxidant; Oxidative stress
Neuroprotective Effect of 7,8-dihydroxyflavone in a Mouse Model of HIV-Associated Neurocognitive Disorder (HAND)
Tapas K Makar1,4#, Joseph Bryant1#, Bosung Shim2, Kaspar Keledjian2, Harry Davis1, Manik Ghosh3, Ajay Koirala1, Ishani Ghosh3, Shreya Makar1, Alonso Heredia1, Malcolm Lane4, J Marc Simard2,6,7, Robert C Gallo1, Volodymyr Gerzanich2* and Istvan Merchenthaler4,5*
1Institute of Human Virology, University of Maryland, School of Medicine, Baltimore, MD 21201, USA
2Department of Neurosurgery, University of Maryland, School of Medicine, Baltimore, MD 21201, USA
3Translational Life Sciences and Technology, University of Maryland, Baltimore County, MD, USA
4Department of Epidemiology and Public Health, University of Maryland, School of Medicine, Baltimore, MD, USA
5Department of Neurobiology, University of Maryland, School of Medicine, Baltimore, MD, USA
6Department of Pathology, University of Maryland, School of Medicine, Baltimore, MD, USA
7Department of Physiology, University of Maryland, School of Medicine, Baltimore, MD, USA
#These authors contributed equally to the work
*Address for Correspondence: Istvan Merchenthaler, Department of Epidemiology and Public Health, University of Maryland, School of Medicine, Baltimore, USA, Email: [email protected]
Volodymyr Gerzanich, Department of Neurosurgery, University of Maryland, School of Medicine, Baltimore, MD 21201, USA, Email: [email protected]
Treatment for HIV-associated neurocognitive disorders (HAND) remains elusive. 7,8-dihydroxyflavone (DHF), an analog of brain-derived neurotrophic factor (BDNF) and a high-affinity TrkB agonist, has been proposed as a viable therapeutic alternative to BDNF in crossing the Blood-Brain Barrier (BBB) and promoting growth, differentiation, maintenance, and survival of neurons. Here, we expand on our previous study investigating the therapeutic role of DHF on the cortical and hippocampal brain regions of the Tg26 mice, an animal model of HAND. We detected increased immunoreactivity for ion channels (SUR1, TRPM4) and the water channel aquaporin-4 (AQP4), suggesting an ionic and osmotic imbalance in the brains of Tg26 mice. Tg26 mice also exhibited loss of synaptic stability (SYN, SYP) and nicotinamide metabolism (NAMPT, SIRT1) that were associated with astrogliosis. Furthermore, Tg26 mice demonstrated increased iNOS and reduced HO-1/NRF2 expressions, implicating increased ER and oxidative stress. DHF treatment in Tg26 mice reversed these pathological changes. These data suggest crosstalk among TrkB, Akt, and related transcription factors (NF-κB, STAT3, and NRF2) as an underlying mechanism of Tg26-associated pathology in the brain. Finally, taken together with our prior study, these results further highlight a therapeutic role of DHF in promoting neuroprotection in HAND that may be applied in conjunction with current antiviral therapies.
HIV: Human Immunodeficiency Virus; HAND: HIV-Associated Neurocognitive Disorder; BDNF: Brain-Derived Neurotrophic Factor; TrkB: Tropomyosin receptor kinase B; DHF: 7,8-Dihydroxyflavone; cART: combined Antiretroviral Therapy; PLWH: People Living with HIV; CNS: Central Nervous System; BBB: Blood Brain Barrier; WT: Wild Type; Tg26: Transgenic Mice 26; DMSO: Dimethyl Sulfoxide; NeuroAIDS: Neuro-Autoimmunodeficiency Syndrome; GFAP: Glial Fibrillary Acidic Protein; SUR1: Sulfonylurea Receptor 1; TRPM4: Transient Receptor Potential Melastatin 4; AQP4: Aquaporin 4; SNP: Synaptophysin; Syp 1: Synapsin 1; NAD: Nicotinamide Adenine Dinucleotide; NAMPT: Nicotinamide Phosphoribosyl Transferase; SIRT1: Sirtuin 1; iNOS: inducible Nitric Oxide Synthase; HO1: Heme Oxygenase 1; Nrf2: Nuclear Factor Erythroid 2-Related Factor 2; STAT-3: Signal Transducer and Activator of Transcription 3; NF-kB: Nuclear Factor Kappa B; Akt: Protein Kinase B; TLR4: Toll-Like Receptor 4; CXCR4: Chemokine Receptor 4; CCR5-C: C Chemokine Receptor Type 5; CSF: Cerebrospinal Fluid Nicotinamide Adenine Dinucleotide; NAMPT: Nicotinamide Phosphoribosyl Transferase; SIRT1: Sirtuin 1; iNOS: inducible Nitric Oxide Synthase; HO1: Heme Oxygenase 1; Nrf2: Nuclear Factor Erythroid 2-Related Factor 2; STAT-3: Signal Transducer and Activator of Transcription 3; NF-kB: Nuclear Factor Kappa B; Akt: Protein Kinase B; TLR4: Toll-Like Receptor 4; CXCR4: Chemokine Receptor 4; CCR5-C: C Chemokine Receptor Type 5; CSF: Cerebrospinal Fluid
The prevalence of severe human immunodeficiency virus (HIV)-associated dementia (HAD) has been decreased by the treatment of combined antiretroviral therapy (cART). On the other hand, the incidence of milder and chronic forms of HIV-associated neurocognitive disorder (HAND) and HIV-associated major depressive disorder is increasing [1]. HIV persists in the brain despite cART [2]. Several studies have reported the toxic effects of HIV-associated viral proteins including gp120, integrase, nef, vpr, and tat within the central nervous system (CNS). Still, conclusive data about the production, release, and associated bystander toxicity are not clearly documented with cART [3,4]. Several animal models have been developed to examine the toxic effects of HIV viral proteins and their role in HIV-associated cognitive impairment [5-7]. Brain-derived neurotrophic factor (BDNF), one of the most abundant growth factors in the brain, is prominently involved in neuronal survival, synaptic transmission, and synaptic plasticity underlying learning and memory through its interaction with the tropomyosin-related kinase B (TrkB) cellular receptor [8-10]. Increased BDNF levels have decreased the odds of developing HAND [10]. Abassi, et al. [11] in a Ugandan population, showed lower cerebrospinal fluid (CSF) BDNF levels in HIV patients with dementia compared with HIV-positive individuals without dementia. Falasca, et al. [12] were able to correlate BDNF levels with different domains of neurocognition. They showed a significant correlation between reduced serum BDNF levels and poor performance on the Grooved Pegboard test for the dominant hand test but no association between BDNF serum levels and attention, executive function, and working memory availability [12]. Processing of pro-BDNF into mature BDNF is reduced in HIV. This contributes to synapto-dendritic injury and synaptic dysfunction seen in HAND. Studies have shown that pro-BDNF levels in HAND subjects were higher than those in HIV-negative as well as HIV-positive subjects without dementia. BDNF reduces the degeneration of synapses and axons triggered by viral proteins. These HIV proteins interact with surface receptors on the neurons or activate caspases to effect neuronal damage. The mechanisms by which these viral proteins induce neurotoxicity include the production of free radicals, nitric oxide, and the release of excitotoxins such as glutamate and inflammatory cytokines [13]. Due to the inability of BDNF to cross the blood-brain barrier (BBB) and its short half-life, efforts have been focused on identifying a small molecule that could mimic the neurotrophic effects of BDNF. Recently, 7,8-diydroxyflavone (DHF), a naturally occurring flavone, was found to be a potent TrkB receptor agonist that mimics the action of BDNF downstream signaling [14]. In vivo mouse studies showed that 7,8-DHF crosses the BBB [15] and causes increased levels of phospho-TrkB protein in the brain [16,17], consistent with the notion that it acts as a BDNF analog and TrkB agonist. Moreover, our studies indicate that 7,8-DHF has beneficial effects in a mouse model of HAND [18]. Others reported beneficial effects of DHF in models of neurodegenerative diseases, such as Huntington’s disease, schizophrenia, and Down Syndrome [19].
In this study, we sought to determine if DHF will exhibit efficacy in preventing or slowing down HAND-related astrogliosis, synaptic plasticity, neuronal metabolic stress, and oxidative stress in a transgenic Tg26 mouse model of HAND. We report that DHF treatment reduced astrogliosis, prevented synaptic degeneration, protected against metabolic changes, and increased the antioxidant capacity in the hippocampus, suggesting that DHF treatment is a promising adjunct therapy with cART.
DHF treatment downregulates activation of astrocytes and Aquaporin 4 in the brain of Tg26 mice
Astrocytes: (GFAP-immunoreactivity): Astrocytes are significant contributors to HIV-1-associated neurological disorders by modulating the microenvironment in the CNS and releasing proinflammatory cytokines [20]. Reactive astrogliosis is a pathological hallmark of HIV infection in brains and is detected by increased glial fibrillary acidic protein (GFAP) staining [21].
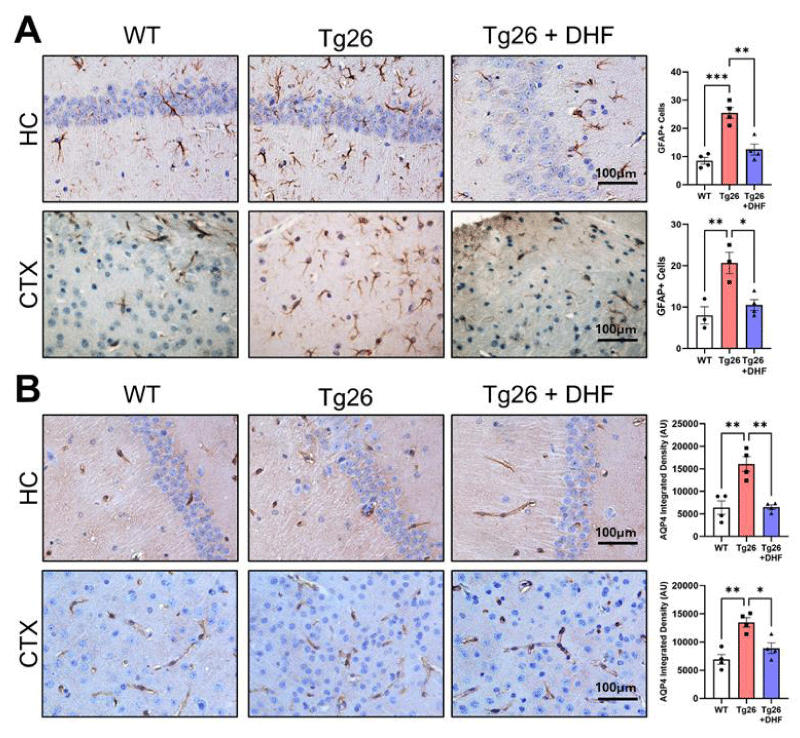
In the present study, GFAP was significantly increased in the hippocampus (p < 0.001) and cortex (p < 0.01) of Tg26 mice, indicative of astrogliosis and DHF significantly decreased GFAP immunoreactivity in the hippocampus (p < 0.01), and cortex (p < 0.05) (Figure 1) of Tg26 mice compared to untreated Tg26 mice. Enhanced astrogliosis before DHF treatment is due to inflammatory molecules enhancing their activities as well as an imbalance of ionic homeostasis. Astroglia dysfunctions are amplified via gap junctions, directly or indirectly impacting surrounding neurons and significantly contributing to the pathogenesis of HIV-associated neuropathology.
Figure 1: Diminished reactive astrogliosis following DHF treatment in Tg26 mouse brains. Representative images of immunohistochemical staining of (A) glial fibrillary acidic protein (GFAP) and (B) the astrocytic water channel aquaporin-4 (AQP4) from the hippocampus (HC) and cortex (CTX) of the following mice: wildtype mice (WT, n = 3 or 4); HIV-associated Tg26 (Tg26, n = 3 or 4); and Tg26 treated with DHF (Tg26+DHF, n = 4). Quantifications from 4-6 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (*** p < 0.001. ** p < 0.01, * p < 0.05).
Aquaporin-4 (AQP4): (Astrocyte immunoreactivity): AQP4 is a water channel expressed on astrocytic end feet in the brain. Increasing evidence suggests that AQP4 is involved in brain inflammation, lymphatic fluid clearance, synaptic plasticity and memory formation, and regulation of extracellular space (ECS) volume and potassium homeostasis [22,23]. The involvement of AQP4 in several pathogenic conditions is mainly based on findings in post-mortem brain tissue, in vitro studies, and the usage of AQP4-deficient rodent models [24].
AQP4 was significantly increased in the hippocampus (p <0.01) and cortex (p < 0.01) of Tg26 mice, indicative of astrogliosis. DHF treatment reduced AQP4 significantly in these brain regions (hippocampus [p < 0.01] and cortex [p < 0.05]) of Tg26 mice compared to Tg26 mice with no treatment (Figure 2). These studies highlight the potential influence AQP4 may have on the neuropathology associated with HAND and suggest that AQP4 expression may be altered in HAND and/or in response to exogenous molecules. Additional studies may be warranted to determine whether altered AQP4 expression represents a protective and/or maladaptive response to CNS inflammation and whether DHF has a neuroprotective role by regulating AQP4.
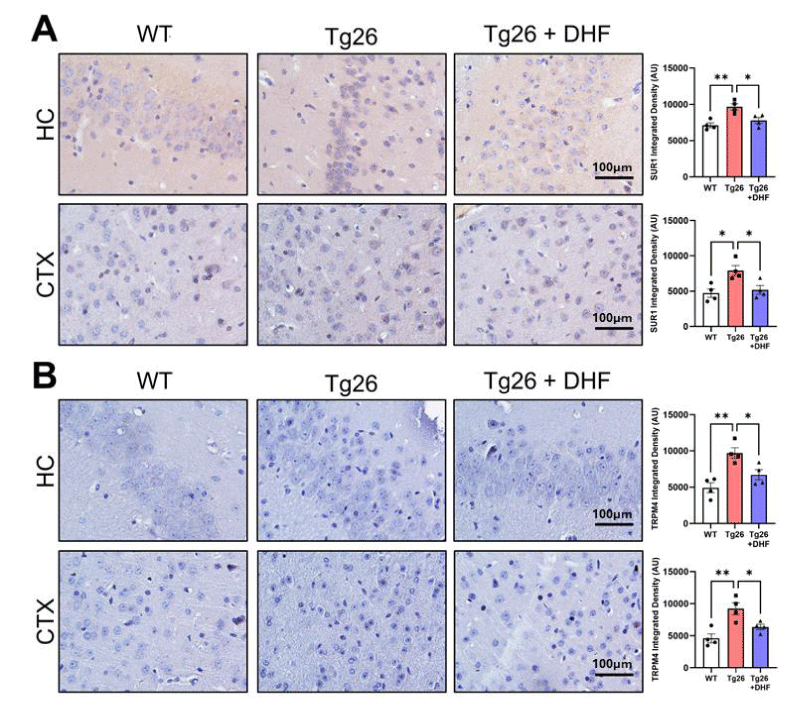
Figure 2: DHF treatment decreases Tg26-induced upregulation of SUR1-TRPM4. Representative images of immunohistochemical staining of (A) sulfonylurea receptor 1 (SUR1) and (B) transient receptor potential cation channel melastatin-4 (TRPM4) from the hippocampus (HC) and cortex (CTX) of wildtype mice: wildtype (WT, n = 4); HIV-associated Tg26 (Tg26, n = 4) mice; and Tg26 mice treated with DHF (Tg26+DHF, n = 4). Quantifications from 5-7 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (** p < 0.01, * p < 0.05).
DHF treatment reduces astrogliosis through SUR1-TRPM4 channel downregulation in the brain of Tg26 mice
SUR1: Sulfonylurea receptor (SUR) belongs to the ATP-Binding Cassette (ABC) transporter family; however, SUR is also associated with ion channels and acts as a regulatory subunit determining the opening or closing of the pore [25]. SUR1-regulated ion channels have been shown to play critical roles as negative regulators of Ca2+ influx.
SUR1 was significantly increased in the hippocampus (p < 0.01) and cortex (p < 0.05) in Tg26 mice and DHF significantly (p < 0.05) decreased it (Figure 3) compared to untreated animals, suggesting again that DHF reduces astrogliosis in these brain regions.
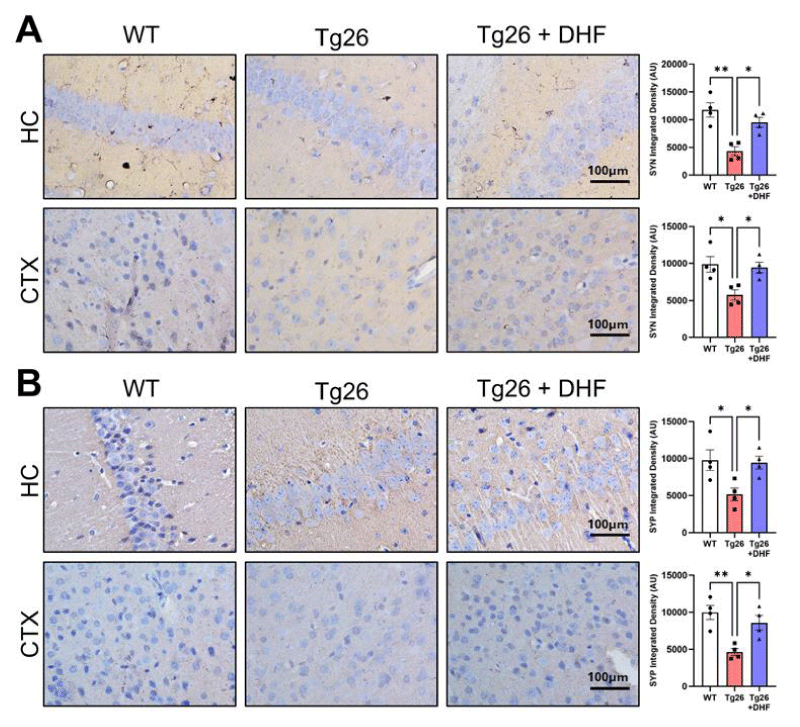
Figure 3: DHF treatment rescues Tg26-induced loss of synaptic stability. Representative images of immunohistochemical staining of (A) synapsin (SYN) and (B) synaptophysin (SYP) from the hippocampus (HC) and cortex (CTX) of wildtype mice (WT, n = 4); HIV-associated Tg26 mice (Tg26, n = 4); and Tg26 mice treated with DHF (Tg26+DHF, n = 4). Quantifications from 6-8 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (** p < 0.01, * p < 0.05).
TRPM4: TRPM4 is a non-selective monovalent cation channel activated by intracellular calcium and modulated by ATP, calmodulin, Protein Kinase-C (PKC), phosphatidylinositol 4,5-bisphosphate, and H2O2 [26]. TRPM4 was significantly (p < 0.01) increased in the hippocampus and cortex of Tg26 mice, confirming astrogliosis, and DHF significantly (p < 0.05) decreased it (Figure 4). These data suggest the importance of recent advances in understanding the role of the SUR1/TRPM4 channel in HAND-associated pathology and DHF-mediated neuroprotection.
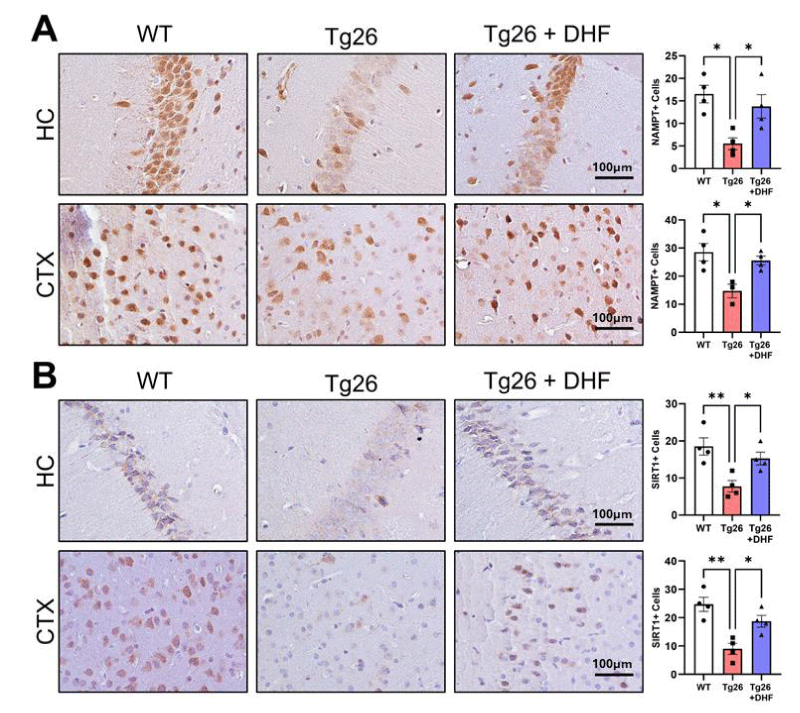
Figure 4: Downregulation of NAD+-related enzymes NAMPT and SIRT1 in Tg26 mouse brains is improved by DHF treatment. Representative images of immunohistochemical staining of (A) nicotinamide phosphoribosyl transferase (NAMPT) and (B) NAD-dependent deacetylase sirtuin-1 (SIRT1) from the hippocampus (HC) and cortex (CTX) of wildtype mice (WT, n = 4); HIV-associated Tg26 mice (Tg26, n = 4); and Tg26 mice treated with DHF (Tg26+DHF, n = 4). Quantifications from 4-7 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (** p < 0.01, * p < 0.05).
DHF treatment upregulates synaptic activity-related proteins in the brain of Tg26 mice
Synapsin-1: Synapsin-1 is a major endogenous substrate for cyclic AMP-dependent protein kinase and for calcium-dependent protein kinase in the presynaptic terminal [27]. At the cellular level, subjects with HAND exhibit synapto-dendritic damage and decreased synaptic and dendritic density [28], which can lead to the interruption of the neural network and ultimately caspase-3-dependent neuronal apoptosis [29]. Tg26 mice exhibited significantly (p < 0.05) decreased Synapsin-1 expression in comparison to the hippocampus and cortex of intact mice (Figure 3A) and DHF treatment significantly (p < 0.05) increased its expression in comparison to Tg26 mice without DHF treatment.
Synaptophysin: Synaptophysin is a 38-kd synaptic vesicle glycoprotein in all types of synaptic vesicle membranes. Synaptophysin is a major synaptic vesicle protein involved in neurotransmitter release. Synaptin-1 and its associated calmodulin-dependent kinase II are additional components of the presynaptic plasma membrane. The main functions of synaptophysin are for docking fusion and endocytosis, otherwise known as membrane trafficking [30,31]. Tg26 mice exhibited significantly decreased Synaptophysin in comparison to intact mice (p < 0.01), and DHF treatment significantly (p < 0.05) increased its expression in the hippocampus and cortex (Figure 3B).
In summary, we identified that synaptic impairment may be contributed to the brain of Tg26 mice, and DHF treatment improved the destruction of synaptic plasticity which is perturbed in HAND.
Downregulation of the NAD+-related enzymes NAMPT and SIRT1 in Tg26 mouse brains is reversed by DHF treatment
NAMPT: Nicotinamide phosphoribosyl transferase (NAMPT) is necessary for the recycling of NAM to NAD, which is part of the salvage pathway of NAD synthesis [32]. Numerous studies have shown that synaptic plasticity is impaired by changes in neurotransmission, immune system malfunction, metabolic dysfunction, and other factors [33]. Among others, lower NAD levels were linked to metabolic and neurodegenerative diseases [34]. NAMPT was significantly (p < 0.05) decreased in the hippocampus and cortex of Tg26 mice, indicative of decreased NAD levels. DHF significantly (p < 0.05) increased NAMPT (Figure 4A) in these brain regions of Tg26 mice. These results suggest that DHF reduces astrogliosis in the hippocampus and cortex of Tg26 mice also by increasing NAD levels.
SIRT1: SIRT1 plays an important role in neurodegenerative diseases as well as in HAND [35,36]. Similar to our previous study [18] in which it was found that SIRT3 expression was downregulated in Tg26 mice, in this study, Tg26 mice also exhibited a significant (p < 0.01) decrease in SIRT1 expression in the hippocampus and cortex in comparison to control mice, and DHF treatment significantly (p < 0.05) increased the expression of SIRT1 in these regions of the brain in comparison to those of the Tg26 mice without DHF treatment (Figure 4B).
Our results also suggest that strategies to augment NAMPT protein expression by upregulation of NAD+ by DHF may have therapeutic benefits to prevent HAND, and the role of SIRT1 during HAND may be important with special reference to HAND-associated pathology and neuroprotection.
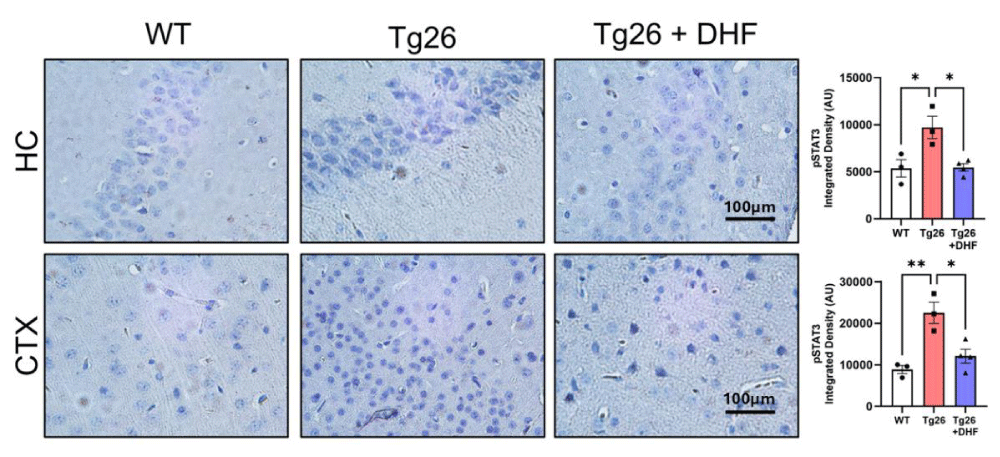
DHF treatment reverses Tg26-induced increased phosphorylation of STAT3
STAT3: The signal transducer and activator of transcription 3 (STAT3) is a pleiotropic molecule that regulates multiple cellular functions. Upon phosphorylation on tyrosine 705 (Tyr705) residue, STAT3 dimerizes and translocates to the nucleus where, it regulates the expression of genes that play a role in cell growth, proliferation, differentiation, and survival [37]. Here, we tested the hypothesis that STAT3 contributes to the orchestration of the antioxidant defense response against the brains of Tg26 mice by DHF treatment (Figure 5). Tg26 mice exhibited significantly increased phosphorylation of STAT-3 in comparison to intact mice in the hippocampus (p < .05) and cortex (p < 0.01) and DHF treatment significantly decreased its expression in these brain regions (p < 0.05). Thus, DHF treatment has neuroprotective effects against HAND via promoting synaptic plasticity through activating TrkB/Akt/STAT3 [18] (as we showed before by activating TrkB/Akt signaling).
Figure 5: DHF treatment reverses Tg26-induced increased phosphorylation of STAT3. Representative images of immunohistochemical staining of phosphorylated STAT3 (pSTAT3) from the hippocampus (HC) and cortex (CTX) of wildtype mice (WT, n = 3); HIV-associated Tg26 mice (Tg26, n = 3); and Tg26 mice treated with DHF (Tg26+DHF, n = 4). Quantifications from 5-8 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (** p < 0.01, * p < 0.05).
DHF treatment ameliorates Tg26-induced ER stress in the brain
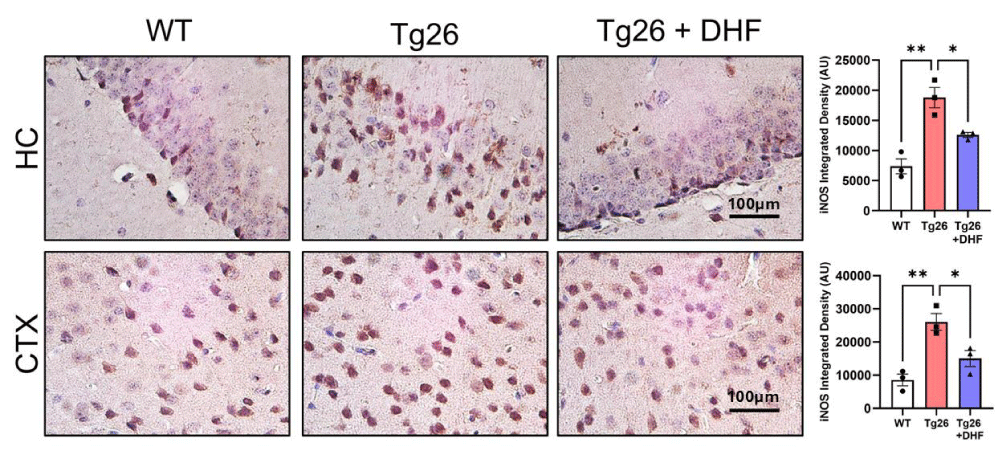
iNOS: Nitric oxide (NO) is synthesized from three different isoforms of nitric oxide synthase (NOS): endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). Among these, iNOS is not usually expressed in the brain. However, activated microglial cells are a major cellular source of iNOS in the brain. The excessive release of NO by activated microglial cells correlates with the progression of neurodegenerative disorders [38,39].
A considerable number of human diseases have an inflammatory component, and a key mediator of immune activation and inflammation is iNOS, which produces NO from L-arginine. Overexpressed or dysregulated iNOS has been implicated in numerous pathologies including HAND [38,39]. We found that in both the hippocampus and cortex of Tg26 mice, iNOS (Figure 6) expression was significantly (p < 0.01) upregulated in comparison to intact mice, and DHF treatment significantly (p < 0.01) downregulated the expression of this enzyme. These results suggest that DHF modulates the expression of iNOS in the hippocampal and cortical regions of Tg26 mice. Further, the data suggest that induction of iNOS in the brain of Tg26 mice may be contributing to HAND-associated pathology. DHF reduces iNOS expression and provides neuroprotection.
Figure 6: DHF treatment ameliorates Tg26-induced ER stress in the brain. Representative images of immunohistochemical stains of inducible nitric oxide synthase (iNOS) from the hippocampus (HC) and cortex (CTX) of wildtype mice (WT, n = 4); HIV-associated Tg26 mice (Tg26, n = 4); and Tg26 mice treated with DHF (Tg26+DHF, n = 4). Quantifications from 6-8 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (** p < 0.01, * p < 0.05).
DHF treatment improves suppressed antioxidant expression in Tg26 mice
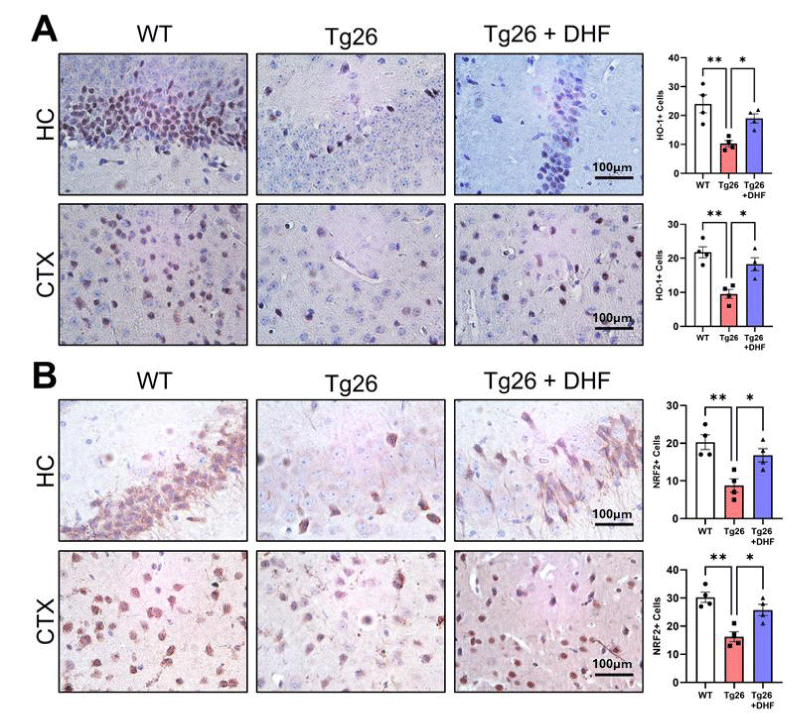
Heme oxygenase 1 (HO-1): The Heme oxygenase-1 (HO-1) system is believed to be a crucial mechanism for the nervous system under stress conditions. HO degrades heme to carbon monoxide, iron, and biliverdin. Several lines of evidence indicate that HO-1 dysregulation is associated with brain inflammation and neurodegeneration, including Parkinson’s and Alzheimer’s disease [40]. We found that in both the hippocampus and cortex of Tg26 mice, HO-1 expression was significantly downregulated (p < 0.01) in comparison to intact mice, and DHF treatment significantly upregulated this enzyme (p < 0.05) expression (Figure 7A). Our data shows that HO-1 induction by DHF treatment could be a therapeutic strategy for neuroprotection against HAND. HO-1 modulates endogenous antioxidant and immune-modulatory pathways, thus, limiting oxidative stress associated with HAND. The use of pharmacological inducers of endogenous HO-1 expression may be a potential adjunctive neuroprotective therapeutics in HAND.
Figure 7: Reduced brain antioxidant expression in Tg26 mice is recovered following DHF treatment. Representative images of immunohistochemical staining of (A) heme oxygenase-1 (HO-1) and (B) nuclear factor erythroid-related factor 2 (NRF2) from the hippocampus (HC) and cortex (CTX) of wildtype mice (WT, n = 4); HIV-associated Tg26 mice (Tg26, n = 4); and Tg26 mice treated with DHF (Tg26+DHF, n = 4). Quantifications from 5-7 immunostained and imaged sections were averaged for each biological replicate. Scale bar = 100 µm. (** p < 0.01, * p < 0.05).
Nrf2: The transcription factor Nrf2 (nuclear factor-erythroid 2 p45-related factor 2) plays a crucial role in cellular redox and metabolic systems. Activation of Nrf2 may be an effective therapeutic approach for neuroinflammatory disorder, through activation of the antioxidant defense system, lowering inflammation, regulating the mitochondrial function, and balancing of protein homeostasis [41]. Tg26 mice exhibited significantly decreased phosphorylation of Nrf2 in comparison to untreated mice (p < 0.01), and DHF treatment significantly (p < 0.05) increased the expression of Nrf-2 in the hippocampus and cortex (Figure 7B). Nrf2 plays a key role in stabilizing the oxidation–antioxidation balance and reducing tissue damage caused by oxidative stress against HAND.
HAND is a serious disease of people living with HIV (PLWH) despite treatment with antiretroviral therapy (ART). Considering the high prevalence of HIV and increased survival rates with ART, it is important to develop treatments for HAND and the resulting clinical deficits. BDNF, an endogenous neuroprotective agent, plays a critical role in brain development, learning, and memory and may play a role in HAND. Lower CSF BDNF levels were found in HIV patients with dementia [11] and could contribute to the neuronal degeneration seen in HAND. A treatment for HAND should be effective, affordable, non-invasive, and have minimal side effects [42]. DHF is a natural analog of BDNF, which is a potent TrkB agonist, and neuroprotective in several animal models of neurodegenerative diseases [11]. We found that DHF injection into Tg26 mice, an animal model of HAND, increased TrkB activation in the cortex and hippocampus of the brain [18]. The work described here expands upon that finding.
We also demonstrated that TLR4, a neuro-inflammatory receptor [18,43] was upregulated in the brains of Tg26 mice and DHF treatment down-regulated the receptor expression. The HIV co-receptors, including CXCR4 and CCR5, play a crucial role even in neuroAIDs [44]. Our previous study demonstrated that these co-receptors were highly expressed in the brains of Tg26 mice and decreased after DHF treatment [18]. This overview below is about what is known and what remains to be explored regarding TrkB, TLR4, co-receptor signaling, and crosstalk between these membrane-bound receptors with special reference to HAND and BDNF-TrkB signaling. Astrocytes are the most abundant glial cells in the CNS. They maintain BBB by providing structure and support as well as serve to maintain the homeostatic balance of molecules and ions, including ATP, calcium, and glutamate within and around the tripartite neuronal synaptic clefts and astrocytes [45,46]. In HAND, dysregulation of astrocytes is well-documented [18,45]. It was shown that HIV-1 expression correlates with the activation of proinflammatory markers (TLR4, TNF-α, and NF-κB) and the SUR1-TRPM4 channel in astrocytes of HIV-infected postmortem human and transgenic Tg26 mouse brain tissues [46]. Furthermore, it was reported that Tg26 mice exhibited impaired cognitive skills and reduced learning abilities compared to wild-type mice, particularly in spatial memory. Interestingly, male Tg26 mice displayed significant differences in spatial memory losses, while no significant differences were identified in female mice [47,48].
Consistent with our early results, SUR1-TRPM4 channels were upregulated in Tg26 mice along with GFAP and AQP4, confirming the role of reactive astrocytes and neuroinflammation in HAND [47]. Interestingly, the present results demonstrate the upregulation of ion channels including SUR1, TRPM4, and AQP4 in addition to astrogliosis in the brains of Tg26 mice together with increased expression of CXCR4, CCR5, and TLR4 together with the decreased TrkB activity [18]. On the other hand, DHF treatment suppressed all those ion channels and astrogliosis together with decreased CXCR4, CCR5, and TLR4 and increased TrkB activation [18]. These findings suggest that TrkB-ligands as well as CXCR4, CCR5, and TLR4 receptors signaling might be involved in the regulation of those ion channels associated with the pathology, and neuroprotection of HAND. These findings also allow us to explore the new concept of ion channel and membrane-bound receptor cross-talks associated with HAND and accompanying pathology.
Now we demonstrated that DHF treatment in Tg26 mice decreased the expression of GFAP, SUR1, TRPM4, and AQP4 in the brain. Earlier, it has been shown that SUR1, TRPM4, and AQP4 made a complex structure in activated astrocytes during CNS injury [49]. It was demonstrated that SUR1-TRPM4-AQP4 transmembrane water influx was altered by increased Ca2+, which is prominent in astrocytes after brain injury [50]. Astrocyte swelling reduces the extracellular space, which impairs the clearance of toxic metabolites [51]; dysregulates the release of glutamate, which contributes to neuronal death [52]; and increases cerebral edema [50], which worsens CNS injury outcomes. Therefore, we could speculate that SUR1, TRPM4, and AQP4 expressions, increased in the brain of Tg26 mice, might suggest transmembrane water influx by unbalancing Na+, K+, and Ca2+ levels. Our earlier findings [47] may indicate that the SUR1-TRPM4-AQP4 complex is particularly important in the HAND-associated pathology.
AQP4 is upregulated in HAND and if AQP4 is dependent on ion channel function, ion channel antagonists could be used to indirectly modulate AQP4. Given that AQP4-specific inhibition has not yet become practical, this is a tempting prospect. Considering our findings, indirect attenuation of AQP4, SUR1, and TRPM4 functions may partially underlie the beneficial effects of DHF-supported neuroprotection mechanisms in HAND. Earlier it has been shown that TrkB activation also modulates ion channels that can alter neuronal excitability, including Na+, Ca2+, and K+ channels through activating signaling pathways [53,54]. For example, BDNF/TrkB activation modulates neuronal excitability by gating Na+ current via Nav1.9 [55]. Furthermore, our data suggest that HIV genes induce proinflammatory responses by activating TLR4, CXCR4, and CCR5 and inhibiting TrkB and are mediated at least in part through the SUR1, TRPM4, and AQP4 channels in astrocytes. Previously, it is reported that TrkB cross-talk with the Kv1.3 potassium channel [56] and CXCR4 and CCR5 interplay with ion channels [57,58]. TLR4 receptors also crosstalk with different ion channels [59]. This is the first time we report the neuroprotective action of DHF in the brain of Tg26 mice by crosstalk between membrane-bound receptors (TrkB, TLR4, CXCR4, and CCR5) and membrane-bound ion channel proteins (SUR1, TRPM4, and AQP4). Furthermore, the central challenge that emerges from our findings is to better understand DHF-mediated neuroprotection in HAND and elucidate the mechanistic and temporal interrelation of the above-mentioned ion channel proteins and membrane-bound receptor proteins.
Synaptic degeneration represents an important histopathological hallmark of HAND [60]. Synaptic dendritic networks in the brain undergo continuous remodeling, a process termed neuroplasticity. Earlier [47], we reported that a reduction in neurosynaptic responses, as indicated by the downregulation of SYN1 and SYP, plays a role in synaptopathy as a possible mechanism associated with cognitive and motor skill deficits. Inhibition of synaptic degeneration therefore provides an attractive therapeutic target to prevent HAND pathogenesis. BDNF is one of the mediators to protect synaptic plasticity [61,62]. Recently, it has been shown that BDNF delivery with nanoparticles enhances neurogenesis, synaptogenesis, and cognitive function in an animal model of neuro-AIDs [63] confirming that a deficit in BDNF/TrkB signaling contributes to the synaptic dysfunction of HAND [10]. We have shown that synaptogenesis (which is determined by SYN1 and SYP) was downregulated in the hippocampus and cortex of Tg26 mice and DHF treatment enhanced synaptogenesis. In our findings, we demonstrated that DHF in Tg26 mice increased synaptic plasticity by inhibiting membrane-bound ion channels (including SUR1, TRPM4, and AQP4) and membrane-bound receptors (TLR4, CXCR4, and CCR5) and the interplay between them results in activation of TrkB [18]. Previously, we have also demonstrated that those ion channel and receptor proteins are involved in neuroinflammation which may be one of the causes of synaptic degeneration [18,47]. It has been shown that activation of TLR4, CXCR4, and CCR5 are involved in regulating synaptic plasticity [64-66]. Importantly, it has been shown that oral administration of DHF enhances synaptic plasticity and prevents memory deficits in an animal model of Alzheimer’s Disease [67]. Furthermore, Zeng, et al. showed that activation of TrkB by NAD prevents fear memory defects and facilitates synaptic plasticity in the amygdala of aging rats [68].
NAD+ is a key cofactor, in cellular energy metabolism [69] and in the modulation of inflammatory signaling [70]. In multiple species, NAD decline with age has been linked to deficits in mitochondrial function and metabolic capacity and a decline in the activity of sirtuins, a class of NAD+-dependent enzymes that control inflammation, and mitochondrial metabolism [71]. Age-related decline of NAD is due in part to hydrolysis by an intrinsic NADase activity of the activation marker CD38 [72]. Defects in NAD+ metabolism have been associated with many neurodegenerative diseases including HAND [73-75]. Tat protein inhibited nicotinamide phosphoribosyltransferase (NAMPT), an enzyme converting NADH to NAD+ [76]. In this context, NAMPT, the rate-limiting factor for NAD synthesis, was decreased in the brain of Tg26 mice and was upregulated by DHF treatment. These results show that HAND-associated neurological dysfunction is coupled with inhibition of NAD+ synthesis and DHF treatment shows its neuroprotective function by increasing NAD+ level. NAD also influences inflammatory signaling, in part, through NAD-dependent SIRT1 deacetylation of the p65 subunit of NF-κB, a heterodimeric transcription factor regulating multiple inflammatory genes [77]. A significant role of SIRT1 in the pathogenesis of neurological disorders in HIV-infected patients [78] has been reported. HIV tat gene also negatively affects SIRT1 activity by affecting the NAD+/NADH ratio, a key factor for the modulation of its activity [76]. This is the first time, that in Tg26 mice, we have found a significant decrease of SIRT1 expression in the hippocampus and cortex compared to WT animals and increased SIRT1 expression by DHF treatment.
Furthermore, we previously observed NF-kB activation in the Tg26 brains and downregulation by DHF treatment [18]. It was reported that an intact intestinal microbiome is required to mediate both, cellular signaling and metabolic protection by increasing SIRT1 elicited by oral DHF in female mice [79]. Occludin is one of the proteins in pericytes, which via the NF-κB/SIRT-1 pathway, modulates HIV-1 transcription, pointing to a significant role of SIRT1 in the pathogenesis of neurological disorders in HIV-infected patients [77,78]. We also found that DHF treatment regulates neurological disorders in Tg26 mice via the NF-kB/SIRT1 pathway. SIRT1 activation via resveratrol has been found to inhibit NF-κB and diminish amyloid-β’s (Aβ) neurotoxic effect in microglia [80]. Furthermore, we determined the roles of signal transducer and activator of transcription 3 (STAT3) in the brain of Tg26 mice before and after DHF treatment. We demonstrated that STAT3 expression was decreased in the brain of Tg26 mice but enhanced by DHF treatment (Figure 5). Previously, it has been shown that STAT3 and its phosphorylation are involved in HIV-1 tat-induced transactivation of GFAP [81]. To date, however, limited information is available about the involvement of STAT3 in HAND pathogenesis. Our recent work has shown that STAT3 downregulation is associated with neurodegeneration and upregulation of STAT3 is involved with neuroprotection associated with DHF treatment. This signaling is involved in TrkB/AKT/SIRT1/NF-kB/STAT3 mediated interplay (as we found TrkB/AKT/NF-kB signaling) [18]. Previously, the neuroprotective role was established by AMPK/SIRT1 and JAK2/STAT3/NF-kB signaling pathway [82]. Furthermore, SIRT1 activity has also demonstrated a neuroprotective role in slowing neurodegenerative disease progression in pathologies such as Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) by upregulating autophagy [83]. SIRT1 has also been shown to be a promising therapeutic target for inhibiting p53 involvement in neurodegenerative diseases [84]. SIRT1 overexpression down-regulates the presence of Aβ and p-tau in the Alzheimer’s disease model while increasing the expression of BDNF [85]. Research shows that SIRT1 is a factor in promoting mitochondrial biogenesis, activating acetyl-CoA synthetase 2 (AceCS2), then AMPK, which in turn phosphorylates and activates PGC-1α [86]. Previously, we found that SIRT3 was also downregulated in the brain of Tg26 mice and DHF treatment enhanced SIRT3 by increasing mitochondrial biogenesis [18]. Furthermore, it has been shown that high levels of SIRT1 expression in the brain exhibited regular synaptic plasticity and memory and it was concluded that SIRT1 is indispensable for normal learning, memory, and synaptic plasticity in mice [87]. Therefore, DHF in our model system balanced metabolic homeostasis, increased mitochondrial biogenesis [18], and showed neuroprotection by enhancing synaptic plasticity via SIRT1 and SIRT3-mediated pathways [84].
Moreover, recent evidence places a key role of chronic inflammation, and more specifically, oxidative stress, and nitrative stress (ROS/RNS) to contribute to the underproduction of antioxidants in HAND-associated pathogenesis [45,88,89]. Reports indicate that HIV infection hastens the normal aging-related neurodegenerative process by accelerating neuronal excitotoxicity, atypical Ca+ signaling, and dysregulated production of ROS/RNS that contribute to oxidative/nitrative stress (OS/NS) [90-92]. This imbalance in the oxidation-reduction (redox) state can be cytotoxic to neurons through the inhibition of cellular respiration, dysregulation of mitochondrial electron transport, and posttranslational modification of neuronal structural proteins [93,94].
We now demonstrate a significant increase in iNOS expression and inflammation [18] in the hippocampus and cortex of Tg26 mice compared to WT mice. On the other hand, DHF treatment reduced the iNOS expression and inflammation [18]. Peroxynitrite and protein nitration appear to be important for HIV-mediated neurodegeneration since the rates and severity of HIV-mediated dementia correlated with the levels of gp41 and iNOS in HIV-1-infected patients [95,96]. These results indicate that HIV-mediated increased nitroxidative stress plays a critical role in contributing to neuroprotection.
To counter the neuroinflammation and oxidative stress, a normal cellular response involves acute induction of HO-1 expression, which generates the potent antioxidants bilirubin and biliverdin, as well as carbon monoxide, which also has pro-survival effects [97,98] by regulating neuroinflammation and oxidative stress. HO-1 may induce the production of glutathione, a potent endogenous antioxidant [98,99], and depletion of glutathione may itself trigger HO-1 induction [97]. HO-1 deficiency was reported in HAND [100]. Thus, HO-1 induction could be another therapeutic strategy for neuroprotection against HAND. Here, we have demonstrated a significant deficiency of HO-1 expression in the hippocampus and cortex and the stimulation of HO-1 expression after DHF treatment in Tg26 mice (Figure 7A). HO-1-modulation of endogenous antioxidant and immune-modulatory pathways, thus limiting the oxidative stress that can promote HIV disease progression in the CNS. Increased HO-1 expression and decreased iNOS expression in the hippocampus from adult spontaneously hypertensive rats have been reported [101], and we also found a decrease in the brain of Tg26 mice after DHF treatment.
Nrf2 is a transcription factor, which is induced by OS/NS and binds to the antioxidant response elements of phase 2 antioxidant enzymes. Nrf2 positively regulates the expression of antioxidants that protect cells from oxidative stress [102]. We also found reduced Nrf2 expression in the hippocampus and cortex of the Tg26 mice which was enhanced by DHF treatment. We also demonstrated that STAT3 an oxidative stress suppressor was upregulated by DHF treatment (Figure 5). Studies have confirmed that Nrf2 activation is accompanied by increased antioxidants and decreased inflammatory cytokine levels, which is expected to reduce cellular oxidative damage [102]. Furthermore, Nrf2/HO-1 is a primary regulator of essential cytoprotective responses in the brain [103]. Downregulation of Nrf2/HO-1 antioxidant signaling is primarily responsible for the development of HAND [102,104]. We have identified downregulation of the Nrf2/HO-1 pathway in the brain of Tg26 mice and upregulation by DHF treatment (Figure 7). It is also reported that HO-1 leads to reduced NF-κB p65/RelA levels [105,106], which may suggest a negative feedback mechanism of NF-κB. Previously, it has been shown that DHF down-regulated NF-κB, iNOS, and caspase-3, and up-regulated the expression of Nrf2, HO-1, and BDNF in the hippocampus [19]. It is demonstrated that DHF exerts anti-oxidation, anti-inflammatory, and anti-apoptotic effects by activating the BDNF-TrkB pathway, thereby improving memory dysfunction induced by alcohol and a high-fat diet [107]. Collectively HAND-induced changes in NF-kb, STAT3, and Nrf2 activities contribute to the pathology of the brain of Tg26 mice, as well as neuroprotection associated with DHF treatment. Crosstalk between the NF-κb, STAT3, and Nrf2 pathways can induce neuronal damage and neuronal protection via regulating ionic homeostasis [46,47], neuroinflammation, metabolic stress, mitochondrial dysfunction/ER stress [18], oxidative stress, and antioxidant defense system. Earlier, many studies highlighted the interplay between HIV and host protein involvement in HIV-mediated neurodegeneration [3,108]. Tg26 mice are the unique pre-clinical model of HAND where the interplay of host and viral proteins are involved with HIV-associated neurotoxicity and DHF-mediated neuroprotection is also associated with protein-protein cross-talks. There is no evidence of clinical trials with DHF and its different derivatives on HAND. It is reported that R13, a prodrug molecule DHF has been tested in a phase 1 clinical trial for Alzheimer’s disease [109]. DHF has been examined from the perspective of pharmacokinetics [110]. In the monkey model of Parkinson’s disease, no toxic effect of DHF was found [111]. Our comprehensive analysis of Tg26 mice brains identifies several proteins and their interactions associated with neurodegeneration and neuroprotection before and after DHF treatment. We have identified the association of various host proteins and neuronal activities with their involvement in multiple pathways responsible for the development of neurotoxicity and neuroprotection. Future studies will be conducted using qPCR and Western blot to elucidate the expression of these targets.
Animals: All animal studies were conducted at the Animal Care Facility of the Institute of Human Virology, University of Maryland, Baltimore, and were approved by The Maryland University Institutional Animal Care and Use Committee. All experiments were conducted by the guidelines and regulations approved by the National Institute of Health. Tg26, an HIV-1 Transgenic mice were used in all experiments. Wild-type (WT) mice with an FVB/N genetic background developed from the same litter of Tg26 mice were used as controls in this study. The generation of transgenic mouse line Tg26 has been described previously [112]. The transgene is derived from the pNL4-3 provirus and contains a 3-kb deletion that spans most of the gag/pol region. Heterozygous mice were used because homozygotes rarely survive to weaning. The colony developing leukemia/lymphoma was generated by crossbreeding heterozygous mice with skin lesions [113]. Mice were euthanized at ages 5–12 mo. All mice were on the FVB/N background.
Drug: 7,8-dihydroxyflavone (DHF) (Tokyo Chemical Industry) was dissolved in 0.2% DMSO/PBS. Mice received one intraperitoneal (i.p.) injection of 7,8-DHF (5 mg/Kg) or vehicle (controls). We chose this dose of DHF as it has been widely used by others and us [18] and shown to improve symptoms in several disease models [114-118]. Mice were divided into 3 groups: untreated control group (3-month-old female Tg26 mice); DHF-treated (3-month-old female Tg26 mice) (Tg + DHF); and Wild type mice (3-month-old female wild-type mice). Tg26 + DHF mice received a daily dose of DHF and controls of vehicle (200 μl of 0.2% DMSO in PBS) for one month.
Immunohistochemistry
Mice of all three groups were euthanized after one month of DHF treatment using the general anesthetic isoflurane/oxygen mixture and perfused with saline and 4% paraformaldehyde. Brains were removed from the skull, immersion-fixed for a day, washed in saline, dehydrated, and embedded in paraffine. 7 μm thick sections containing the hippocampus and cerebral cortex were prepared for immunohistochemical staining. Staining methods are described previously [18]. Histological quantification was performed by a blind observer using Image J. All cell labeling experiments (antibodies listed in Table 1) were quantified based on the number of positive cells/fields.
| Table 1: List and concentration of antibodies used in the study. | ||||
| Antibody | Target | Vendor / Cat # | Dilution | Poly/monoclonal |
| Anti-SUR1 | Sulfonylurea receptor- 1 | Custom made - NA | 1:250 | Polyclonal |
| Anti-TRPM4 | Transient receptor Potential Cation Channel Subfamily M Member 4 | Millipore Sigma - ABn418 | 1:200 | Polyclonal |
| Anti-GFAP | Glial fibrillary acidic protein | Millipore Sigma - AB5541 | 1:100 | Polyclonal |
| Anti- AQP4 | Aquaporin 4 | Santa Cruz Biotechnology - sc-32739 | 1:100 | Monoclonal |
| Anti- Synapsin-1 | Synapsin-1 / synaptic marker | Biosensis – NR-1822 | 1:200 | Polyclonal |
| Anti- Synaptophysin | Synaptophysin / synaptic maker | Novus Biologicals – NB300-653 | 1:200 | Polyclonal |
| Anti-pSTAT3 | Phosphorylated Stat3 transcription factor | Cell Signaling Technology - 9145 | 1:50 | Monoclonal |
| Anti-SIRT1 | Sirtuin family class I | LifeSpan Bioscience – 11748-MM04 | 1:500 | Monoclonal |
| Ant-NAMPT | Visfatin, PBEF | Novus Biologicals – NBP276-368 | 1:100 | Monoclonal |
| Anti-Nrf2 | Oxidative stress | Santa Cruz Biotechnology SC:365949 | 1:500 | Monoclonal |
| Anti-HO-1 | Heme oxygenase 1 | Santa Cruz Biotechnology SC:390991 | 1:500 | Monoclonal |
| Anti-iNOS | Inducible nitric oxide synthase | Novus Biologicals – NB300-605 | 1:200 | Polyclonal |
Statistical analysis
Statistical analyses were performed, and data were plotted using the GraphPad Prism software (version 9.5.1, GraphPad, San Diego, CA). Data for each group of mice (WT, Tg26, Tg26 + DHF) are presented as means ± SEM. Each data point per group represents one mouse as a biological replicate, with quantification averaged from multiple immunostained sections specified in the figure legends. Statistical analyses were performed using the one-way ANOVA followed by Bonferroni’s multiple comparison post hoc test to compare each pair of groups. Statistical significance was accepted at p < 0.05 and is indicated in the plotted data (*p < 0.05, **p < 0.01, ***p < 0.001).
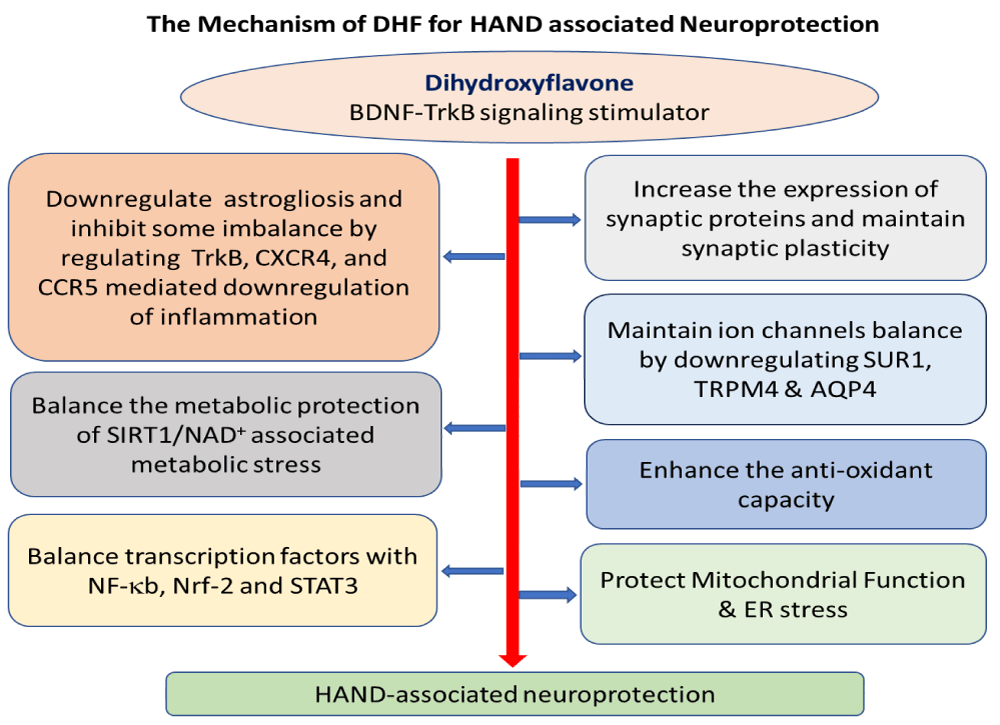
In conclusion, this study provides new evidence for the molecular mechanisms and signaling pathways triggered by DHF treatment in the brain of Tg26 mice, an animal model of HAND, emphasizing the role of BDNF-TrkB signaling [18] as well as other signaling pathways in contributing to viral pathogenesis and neuroprotection. Figure 8 describes the potential mechanisms or crosstalk of mechanisms associated with DHF treatment in regulating neuroprotection in HAND. These findings define the association between brain HIV-1 infection and the involvement of the interplay of several proteins related to HAND and neuroprotection and establish a proof-of-concept therapeutic link to HIV induction as an adjunctive approach for HAND-associated neuroprotection with DHF treatment. Given the powerful therapeutic efficacy of DHF in preclinical animal models, it is reasonable to believe that DHF has a potential future in clinical trials for HAND.
Figure 8: Proposed mechanism of DHF improving neurodegeneration in the brain of Tg26 mice. DHF may be acting through the BDNF-TrkB and downstream signaling pathways such as Akt, NF-κB, Nrf-2, and crosstalk with STAT3 (TrkB, Akt, and NF-κB) in various brain regions [18]. Through this signaling pathway, DHF may increase synaptic proteins in hippocampal and cortical regions, retain synaptic structure and function, protect SIRT1/NAD+-associated metabolic stress, and preserve mitochondrial function [18] by activating anti-inflammatory properties and enhancing antioxidant capacity. As a result, DHF may also reduce astrogliosis and expression of membrane-bound ion channels (SUR1, TRPM4, and AQP4) and receptor (TrkB, CXCR4, and CCR5) crosstalk in the brain [18].
We thank Sumiko Williams for caring for and maintaining the animals. We also thank Dr. Shyamasundaran Kottilil and Dr. Christopher Bever for reviewing and commenting on the manuscript.
Funding
This work is supported in part by NIH Grant R01NS107262 NINDS (V.G.) and a Departmental grant within the Institute of Human Virology, Baltimore, MD. These funding bodies were utilized for obtaining the animals used in the study and covering the article processing and publishing fees.
Author contributions
T.K.M., J.B., V.G., and I.M. conceptualized, designed, and supervised the experiments. J.B. provided the mouse strain. H.D. maintained the animals up to the sample stage. M.L. prepared the samples. A.J., S.M., M.L., I.G., A.H., and M.G. performed the IHC staining. A.K. and B.S. imaged the stained samples. B.S. and K.K. performed data analyses and designed the figures, T.K.M., J.B., V.G., and I.M. interpreted the results and wrote the manuscript. V.G., J.M.S., R.C.G., and I.M. commented on the manuscript, offered revisions, and assisted with project administration.
- Tedaldi EM, Minniti NL, Fischer T. HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int. 2015;2015:5641913. Available from: https://doi.org/10.1155/2015/641913
- Sun W, Rassadkina Y, Gao C, Collens SI, Lian X, Solomon IH, et al. Persistence of intact HIV-1 proviruses in the brain during antiretroviral therapy. Elife. 2023;12. Available from: https://doi.org/10.7554/elife.89837
- Jadhav S, Nema V. HIV-Associated Neurotoxicity: The Interplay of Host and Viral Proteins. Mediators Inflamm. 2021;2021:1267041. Available from: https://doi.org/10.1155%2F2021%2F1267041
- Sanchez AB, Kaul M. Neuronal Stress and Injury Caused by HIV-1, cART and Drug Abuse: Converging Contributions to HAND. Brain Sci. 2017;7(3):33. Available from: https://doi.org/10.3390%2Fbrainsci7030025
- Evans DT, Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013;8(4):255-61. Available from: https://doi.org/10.1097/coh.0b013e328361cee8
- Su H, Cheng Y, Sravanam S, Mathews S, Gorantla S, Poluektova LY, et al. Immune Activations and Viral Tissue Compartmentalization During Progressive HIV-1 Infection of Humanized Mice. Front Immunol. 2019;10:340. Available from: https://doi.org/10.3389%2Ffimmu.2019.00340
- Bar KJ, Coronado E, Hensley-McBain T, O'Connor MA, Osborn JM, Miller C, et al. Simian-Human Immunodeficiency Virus SHIV.CH505 Infection of Rhesus Macaques Results in Persistent Viral Replication and Induces Intestinal Immunopathology. J Virol. 2019;93(18). Available from: https://doi.org/10.1128%2FJVI.00372-19
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5(11):1177-84. Available from: https://doi.org/10.1038/nn927
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331-7. Available from: https://doi.org/10.1038/nm.1912
- Michael H, Mpofana T, Ramlall S, Oosthuizen F. The Role of Brain Derived Neurotrophic Factor in HIV-Associated Neurocognitive Disorder: From the Bench-Top to the Bedside. Neuropsychiatr Dis Treat. 2020;16:3557-67. Available from: https://doi.org/10.2147%2FNDT.S232836
- Abassi M, Morawski BM, Nakigozi G, Nakasujja N, Kong X, Meya DB, et al. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J Neurovirol. 2017;23(3):369-75. Available from: https://doi.org/10.1007%2Fs13365-016-0505-9
- Falasca K, Reale M, Ucciferri C, Di Nicola M, Di Martino G, D'Angelo C, et al. Cytokines, Hepatic Fibrosis, and Antiretroviral Therapy Role in Neurocognitive Disorders HIV Related. AIDS Res Hum Retroviruses. 2017;33(3):246-53. Available from: https://doi.org/10.1089/aid.2016.0138
- Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379-87. Available from: https://www.nature.com/articles/aps200924
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687-92. Available from: https://doi.org/10.1073/pnas.0913572107
- Liu X, Qi Q, Xiao G, Li J, Luo HR, Ye K. O-methylated metabolite of 7,8-dihydroxyflavone activates TrkB receptor and displays antidepressant activity. Pharmacology. 2013;91(3-4):185-200. Available from: https://doi.org/10.1159%2F000346920
- Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22(3):399-408. Available from: https://doi.org/10.1002/hipo.20906
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry. 2011;168(2):163-72. Available from: https://doi.org/10.1176/appi.ajp.2010.10030326
- Bryant J, Andhavarapu S, Bever C, Guda P, Katuri A, Gupta U, et al. 7,8-Dihydroxyflavone improves neuropathological changes in the brain of Tg26 mice, a model for HIV-associated neurocognitive disorder. Sci Rep. 2021;11(1):18519. Available from: https://doi.org/10.1038/s41598-021-97220-8
- Yang S, Zhu G. 7,8-Dihydroxyflavone and Neuropsychiatric Disorders: A Translational Perspective from the Mechanism to Drug Development. Curr Neuropharmacol. 2022;20(8):1479-97. Available from: https://doi.org/10.2174/1570159x19666210915122820
- Ton H, Xiong H. Astrocyte Dysfunctions and HIV-1 Neurotoxicity. J AIDS Clin Res. 2013;4(11):255. Available from: https://doi.org/10.4172%2F2155-6113.1000255
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7-35. Available from: https://doi.org/10.1007/s00401-009-0619-8
- Hubbard JA, Szu JI, Binder DK. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull. 2018;136:118-29. Available from: https://doi.org/10.1016/j.brainresbull.2017.02.011
- Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012;11(6):535-44. Available from: https://doi.org/10.1016/s1474-4422(12)70133-3
- Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758(8):1085-93. Available from: https://doi.org/10.1016/j.bbamem.2006.02.018
- Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32(9):1699-717. Available from: https://doi.org/10.1038%2Fjcbfm.2012.91
- Cho CH, Lee YS, Kim E, Hwang EM, Park JY. Physiological functions of the TRPM4 channels via protein interactions. BMB Rep. 2015;48(1):1-5. Available from: https://doi.org/10.5483%2FBMBRep.2015.48.1.252
- Song SH, Augustine GJ. Synapsin Isoforms and Synaptic Vesicle Trafficking. Mol Cells. 2015;38(11):936-40. Available from: https://doi.org/10.14348%2Fmolcells.2015.0233
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33-44. Available from: https://doi.org/10.1038/nrn2040
- James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, et al. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25(5):380-6. Available from: https://doi.org/10.1046/j.1365-2990.1999.00195.x
- Zucker RS, Bennett M. Release of neurotransmitters. In: Zigmond MJ, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego: Academic Press; 1999;155-92.
- Sarnat HB, Flores-Sarnat L, Trevenen CL. Synaptophysin immunoreactivity in the human hippocampus and neocortex from 6 to 41 weeks of gestation. J Neuropathol Exp Neurol. 2010;69(3):234-45. Available from: https://doi.org/10.1097/nen.0b013e3181d0151f
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279(49):50754-63. Available from: https://doi.org/10.1074/jbc.m408388200
- Lepeta K, Lourenco MV, Schweitzer BC, Martino Adami PV, Banerjee P, Catuara-Solarz S, et al. Synaptopathies: synaptic dysfunction in neurological disorders - A review from students to students. J Neurochem. 2016;138(6):785-805. Available from: https://doi.org/10.1111/jnc.13713
- Vreones M, Mustapic M, Moaddel R, Pucha KA, Lovett J, Seals DR, et al. Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell. 2023;22(1). Available from: https://doi.org/10.1111/acel.13754
- Fujita Y, Yamashita T. Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front Neurosci. 2018;12:778. Available from: https://doi.org/10.3389%2Ffnins.2018.00778
- Figarola-Centurion I, Escoto-Delgadillo M, Gonzalez-Enriquez GV, Gutierrez-Sevilla JE, Vazquez-Valls E, Torres-Mendoza BM. Sirtuins Modulation: A Promising Strategy for HIV-Associated Neurocognitive Impairments. Int J Mol Sci. 2022;23(2):653. Available from: https://doi.org/10.3390/ijms23020643
- Chang Z, Wang Y, Zhou X, Long JE. STAT3 roles in viral infection: antiviral or proviral? Future Virol. 2018;13(8):557-74. Available from: https://doi.org/10.2217%2Ffvl-2018-0033
- Picon-Pages P, Garcia-Buendia J, Munoz FJ. Functions and dysfunctions of nitric oxide in brain. Biochim Biophys Acta Mol Basis Dis. 2019;1865(8):1949-67. Available from: https://doi.org/10.1016/j.bbadis.2018.11.007
- Vincent VA, De Groot CJ, Lucassen PJ, Portegies P, Troost D, Tilders FJ, et al. Nitric oxide synthase expression and apoptotic cell death in brains of AIDS and AIDS dementia patients. AIDS. 1999;13(3):317-26. Available from: https://doi.org/10.1097/00002030-199902250-00003
- Nitti M, Piras S, Brondolo L, Marinari UM, Pronzato MA, Furfaro AL. Heme Oxygenase 1 in the Nervous System: Does It Favor Neuronal Cell Survival or Induce Neurodegeneration? Int J Mol Sci. 2018;19(8):2354. Available from: https://doi.org/10.3390%2Fijms19082260
- Singh S, Nagalakshmi D, Sharma KK, Ravichandiran V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: A review. Heliyon. 2021;7(2). Available from: https://doi.org/10.1016%2Fj.heliyon.2021.e06216
- Tang H, Lu D, Pan R, Qin X, Xiong H, Dong J. Curcumin improves spatial memory impairment induced by human immunodeficiency virus type 1 glycoprotein 120 V3 loop peptide in rats. Life Sci. 2009;85(1-2):1-10. Available from: https://doi.org/10.1016/j.lfs.2009.03.013
- Souza-Junior FJC, Lisboa SF. Toll-like receptor 4 in the interface between neuroimmune response and behavioral alterations caused by stress. Exploration of Neuroprotective Therapy. Exploration of Neuroprotective Therapy. 2022;(2):182-209. Available from: https://doi.org/10.37349/ent.2022.00028
- Nickoloff-Bybel EA, Festa L, Meucci O, Gaskill PJ. Co-receptor signaling in the pathogenesis of neuroHIV. Retrovirology. 2021;18(1):24. Available from: https://retrovirology.biomedcentral.com/articles/10.1186/s12977-021-00569-x
- Wahl A, Al-Harthi L. HIV infection of non-classical cells in the brain. Retrovirology. 2023;20(1):1. Available from: https://retrovirology.biomedcentral.com/articles/10.1186/s12977-023-00616-9
- Li G, Makar T, Gerzanich V, Kalakonda S, Ivanova S, Pereira EFR, et al. HIV-1 Vpr-Induced Proinflammatory Response and Apoptosis Are Mediated through the SUR1-TRPM4 Channel in Astrocytes. mBio. 2020;11(6). Available from: https://doi.org/10.1128/mbio.02939-20
- Keledjian K, Makar T, Zhang C, Zhang J, Shim B, Davis H, et al. Correlation of HIV-Induced Neuroinflammation and Synaptopathy with Impairment of Learning and Memory in Mice with HAND. J Clin Med. 2023;12(16). Available from: https://doi.org/10.3390%2Fjcm12165169
- Barbe MF, Loomis R, Lepkowsky AM, Forman S, Zhao H, Gordon J. A longitudinal characterization of sex-specific somatosensory and spatial memory deficits in HIV Tg26 heterozygous mice. PLoS One. 2020;15(12). Available from: https://doi.org/10.1371/journal.pone.0244725
- Stokum JA, Kurland DB, Gerzanich V, Simard JM. Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res. 2015;40(2):317-28. Available from: https://doi.org/10.1007/s11064-014-1374-3
- Stokum JA, Kwon MS, Woo SK, Tsymbalyuk O, Vennekens R, Gerzanich V, et al. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia. 2018;66(1):108-25. Available from: https://doi.org/10.1002%2Fglia.23231
- Verkhratsky A, Butt A, Li B, Illes P, Zorec R, Semyanov A, et al. Astrocytes in human central nervous system diseases: a frontier for new therapies. Signal Transduct Target Ther. 2023;8(1):396. Available from: https://www.nature.com/articles/s41392-023-01628-9
- Mahmoud S, Gharagozloo M, Simard C, Gris D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells. 2019;8(2):234. Available from: https://doi.org/10.3390%2Fcells8020184
- Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature. 2002;419(6908):687-93. Available from: https://doi.org/10.1038/nature01085
- Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol. 2002;542(Pt 2):413-29. Available from: https://doi.org/10.1113/jphysiol.2002.017376
- Colley BS, Biju KC, Visegrady A, Campbell S, Fadool DA. Neurotrophin B receptor kinase increases Kv subfamily member 1.3 (Kv1.3) ion channel half-life and surface expression. Neuroscience. 2007;144(2):531-46. Available from: https://doi.org/10.1016/j.neuroscience.2006.09.055
- Colley BS, Cavallin MA, Biju K, Marks DR, Fadool DA. Brain-derived neurotrophic factor modulation of Kv1.3 channel is disregulated by adaptor proteins Grb10 and nShc. BMC Neurosci. 2009;10:108. Available from: https://doi.org/10.1186%2F1471-2202-10-8
- Liu QH, Williams DA, McManus C, Baribaud F, Doms RW, Schols D, et al. HIV-1 gp120 and chemokines activate ion channels in primary macrophages through CCR5 and CXCR4 stimulation. Proc Natl Acad Sci U S A. 2000;97(9):4832-7. Available from: https://doi.org/10.1073%2Fpnas.090521697
- Lin G, Baribaud F, Romano J, Doms RW, Hoxie JA. Identification of gp120 binding sites on CXCR4 by using CD4-independent human immunodeficiency virus type 2 Env proteins. J Virol. 2003;77(2):931-42. Available from: https://doi.org/10.1128%2FJVI.77.2.931-942.2003
- Cosme D, Soares-da-Silva P, Magro F. Effect of Toll-like receptor-2, -4, -5, -7, and NOD2 stimulation on potassium channel conductance in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2022;323(5). Available https://doi.org/10.1152/ajpgi.00139.2022
- Di Liberto G, Egervari K, Kreutzfeldt M, Schurch CM, Hewer E, Wagner I, et al. Neurodegenerative phagocytes mediate synaptic stripping in Neuro-HIV. Brain. 2022;145(8):2730-41. Available from: https://doi.org/10.1093/brain/awac102
- Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front Cell Neurosci. 2019;13:363. Available from: https://doi.org/10.3389%2Ffncel.2019.00363
- Bachis A, Adams AV, Lim ST, Mocchetti I. Neurotrophic Factors and NeuroAIDS: A Lesson from Brain-Derived Neurotrophic Factor. New York, NY: Springer. 2014.
- Vitaliano GD, Kim JK, Kaufman MJ, Adam CW, Zeballos G, Shanmugavadivu A, et al. Clathrin-nanoparticles deliver BDNF to hippocampus and enhance neurogenesis, synaptogenesis and cognition in HIV/neuroAIDS mouse model. Commun Biol. 2022;5(1):236. Available from: https://doi.org/10.1038/s42003-022-03177-3
- Rosa JM, Farre-Alins V, Ortega MC, Navarrete M, Lopez-Rodriguez AB, Palomino-Antolin A, et al. TLR4 pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. Br J Pharmacol. 2021;178(17):3395-413. Available from: https://doi.org/10.1111/bph.15488
- Friedman-Levi Y, Liraz-Zaltsman S, Shemesh C, Rosenblatt K, Kesner EL, Gincberg G, et al. Pharmacological blockers of CCR5 and CXCR4 improve recovery after traumatic brain injury. Exp Neurol. 2021;338:113604. Available from: https://doi.org/10.1016/j.expneurol.2021.113604
- Necula D, Riviere-Cazaux C, Shen Y, Zhou M. Insight into the roles of CCR5 in learning and memory in normal and disordered states. Brain Behav Immun. 2021;92:1-9. Available from: https://doi.org/10.1016/j.bbi.2020.11.037
- Zhang Z, Liu X, Schroeder JP, Chan CB, Song M, Yu SP, et al. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2014;39(3):638-50. Available from: https://doi.org/10.1038/npp.2013.243
- Zeng Y, Liu Y, Wu M, Liu J, Hu Q. Activation of TrkB by 7,8-dihydroxyflavone prevents fear memory defects and facilitates amygdalar synaptic plasticity in aging. J Alzheimers Dis. 2012;31(4):765-78. Available from: https://doi.org/10.3233/jad-2012-120886
- Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22(1):31-53. Available from: https://doi.org/10.1016%2Fj.cmet.2015.05.023
- Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019;28(7):1717-28.e6. Available from: https://doi.org/10.1016/j.celrep.2019.07.043
- Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27(3):529-47. Available from: https://doi.org/10.1016%2Fj.cmet.2018.02.011
- Camacho-Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23(6):1127-39. Available from: https://doi.org/10.1016/j.cmet.2016.05.006
- Brakedal B, Dolle C, Riemer F, Ma Y, Nido GS, Skeie GO, et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson's disease. Cell Metab. 2022;34(3):396-407.e6. Available from: https://doi.org/10.1016/j.cmet.2022.02.001
- Li C, Wu LE. Risks and rewards of targeting NAD(+) homeostasis in the brain. Mech Ageing Dev. 2021;198:111545. Available from: https://doi.org/10.1016/j.mad.2021.111545
- Groth B, Venkatakrishnan P, Lin SJ. NAD(+) Metabolism, Metabolic Stress, and Infection. Front Mol Biosci. 2021;8:686412. Available from: https://doi.org/10.3389/fmolb.2021.686412
- Chen XY, Zhang HS, Wu TC, Sang WW, Ruan Z. Down-regulation of NAMPT expression by miR-182 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation. Int J Biochem Cell Biol. 2013;45(2):292-8. Available from: https://doi.org/10.1016/j.biocel.2012.11.002
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369-80. Available from: https://doi.org/10.1038/sj.emboj.7600244
- Castro V, Bertrand L, Luethen M, Dabrowski S, Lombardi J, Morgan L, et al. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J. 2016;30(3):1234-46. Available from: https://doi.org/10.1096/fj.15-277673
- Sharma P, Silva C, Pfreundschuh S, Ye H, Sampath H. Metabolic protection by the dietary flavonoid 7,8-dihydroxyflavone requires an intact gut microbiome. Front Nutr. 2022;9:987956. Available from: https://doi.org/10.3389%2Ffnut.2022.987956
- Gambini J, Ingles M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, et al. Properties of Resveratrol: in vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid Med Cell Longev. 2015;2015:837042. Available from: https://doi.org/10.1155/2015/837042
- Fan Y, Timani KA, He JJ. STAT3 and its phosphorylation are involved in HIV-1 Tat-induced transactivation of glial fibrillary acidic protein. Curr HIV Res. 2015;13(1):55-63. Available from: https://doi.org/10.2174%2F1570162x13666150121115804
- Yan J, Tang X, Zhou ZQ, Zhang J, Zhao Y, Li S, et al. Sirtuins functions in central nervous system cells under neurological disorders. Front Physiol. 2022;13:886087. Available from: https://doi.org/10.3389%2Ffphys.2022.886087
- Song Y, Wu Z, Zhao P. The protective effects of activating Sirt1/NF-kappaB pathway for neurological disorders. Rev Neurosci. 2022;33(4):427-38. Available from: https://doi.org/10.1515/revneuro-2021-0118
- Wang J, Zheng B, Yang S, Zhou D, Wang J. Olmesartan Prevents Oligomerized Amyloid beta (Abeta)-Induced Cellular Senescence in Neuronal Cells. ACS Chem Neurosci. 2021;12(7):1162-9. Available from: https://doi.org/10.1021/acschemneuro.0c00775
- Corpas R, Revilla S, Ursulet S, Castro-Freire M, Kaliman P, Petegnief V, et al. SIRT1 Overexpression in Mouse Hippocampus Induces Cognitive Enhancement Through Proteostatic and Neurotrophic Mechanisms. Mol Neurobiol. 2017;54(7):5604-19. Available from: https://doi.org/10.1007/s12035-016-0087-9
- Zhou Y, Wang S, Li Y, Yu S, Zhao Y. SIRT1/PGC-1alpha Signaling Promotes Mitochondrial Functional Recovery and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front Mol Neurosci. 2017;10:443. Available from: https://doi.org/10.3389/fnmol.2017.00443
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695-707. Available from: https://doi.org/10.1523%2FJNEUROSCI.0027-10.2010
- Louboutin JP, Strayer D. Role of Oxidative Stress in HIV-1-Associated Neurocognitive Disorder and Protection by Gene Delivery of Antioxidant Enzymes. Antioxidants (Basel). 2014;3(4):770-97. Available from: https://doi.org/10.3390%2Fantiox3040770
- Shah S, Maric D, Denaro F, Ibrahim W, Mason R, Kumar A, et al. Nitrosative Stress Is Associated with Dopaminergic Dysfunction in the HIV-1 Transgenic Rat. Am J Pathol. 2019;189(7):1375-85. Available from: https://doi.org/10.1016%2Fj.ajpath.2019.03.004
- Goodkin K, Miller EN, Cox C, Reynolds S, Becker JT, Martin E, et al. Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study. Lancet HIV. 2017;4(9). Available from: https://doi.org/10.1016/s2352-3018(17)30098-x
- Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. J Clin Epidemiol. 2001;54 Suppl 1. Available https://doi.org/10.1016/s0895-4356(01)00445-0
- Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E. Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol. 2014;9(2):102-16. Available from: https://doi.org/10.1007/s11481-013-9520-2
- Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88(Pt B):179-88. Available from: https://doi.org/10.1016%2Fj.freeradbiomed.2015.04.036
- Cassina A, Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328(2):309-16. Available from: https://doi.org/10.1006/abbi.1996.0178
- Torre D, Pugliese A, Speranza F. Role of nitric oxide in HIV-1 infection: friend or foe? Lancet Infect Dis. 2002;2(5):273-80. Available from: https://doi.org/10.1016/s1473-3099(02)00262-1
- Hori K, Burd PR, Furuke K, Kutza J, Weih KA, Clouse KA. Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood. 1999;93(6):1843-50. Available from: https://pubmed.ncbi.nlm.nih.gov/10068656/
- Ryter SW, Choi AM. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res. 2016;167(1):7-34. Available from: https://doi.org/10.1016/j.trsl.2015.06.011
- Srisook K, Cha YN. Super-induction of HO-1 in macrophages stimulated with lipopolysaccharide by prior depletion of glutathione decreases iNOS expression and NO production. Nitric Oxide. 2005;12(2):70-9. Available from: https://doi.org/10.1016/j.niox.2004.12.002
- Srisook K, Kim C, Cha YN. Cytotoxic and cytoprotective actions of O2- and NO (ONOO-) are determined both by cellular GSH level and HO activity in macrophages. Methods Enzymol. 2005;396:414-24. Available from: https://doi.org/10.1016/s0076-6879(05)96035-7
- Ambegaokar SS, Kolson DL. Heme oxygenase-1 dysregulation in the brain: implications for HIV-associated neurocognitive disorders. Curr HIV Res. 2014;12(3):174-88. Available from: https://doi.org/10.2174%2F1570162X12666140526122709
- Huang Y, Wu L, Xu C, Yang B, Wang R. Increased HO-1 expression and decreased iNOS expression in the hippocampus from adult spontaneously hypertensive rats. Cell Biochem Biophys. 2006;46(1):35-42. Available from: https://link.springer.com/article/10.1385/CBB:46:1:35
- Han D, Lu X, Yin W, Fu H, Zhang X, Cheng L, et al. Activation of NRF2 blocks HIV replication and apoptosis in macrophages. Heliyon. 2023;9(1). Available from: https://doi.org/10.1016%2Fj.heliyon.2022.e12575
- Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678-92. Available from: https://doi.org/10.1093/brain/awq386
- Gill AJ, Kovacsics CE, Cross SA, Vance PJ, Kolson LL, Jordan-Sciutto KL, et al. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest. 2014;124(10):4459-72. Available from: https://doi.org/10.1172/jci72279
- Roach JP, Moore EE, Partrick DA, Damle SS, Silliman CC, McIntyre RC Jr, et al. Heme oxygenase-1 induction in macrophages by a hemoglobin-based oxygen carrier reduces endotoxin-stimulated cytokine secretion. Shock. 2009;31(3):251-7. Available from: https://doi.org/10.1097/shk.0b013e3181834115
- Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, et al. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int. 2005;68(2):611-22. Available from: https://doi.org/10.1111/j.1523-1755.2005.00439.x
- Pandey SN, Kwatra M, Dwivedi DK, Choubey P, Lahkar M, Jangra A. 7,8-Dihydroxyflavone alleviated the high-fat diet and alcohol-induced memory impairment: behavioral, biochemical and molecular evidence. Psychopharmacology (Berl). 2020;237(6):1827-40. Available from: https://doi.org/10.1007/s00213-020-05502-2
- Wang S, Ding X, Li Z, Rao F, Xu H, Lu J, et al. Comprehensive analyses identify potential biomarkers for encephalitis in HIV infection. Sci Rep. 2023;13(1):18418. Available from: https://doi.org/10.1038/s41598-023-45922-6
- Chen C, Ahn EH, Kang SS, Liu X, Alam A, Ye K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPbeta/AEP signaling activation in Alzheimer's disease mouse model. Sci Adv. 2020;6(31). Available from: https://doi.org/10.1126/sciadv.aba0466
- He J, Xiang Z, Zhu X, Ai Z, Shen J, Huang T, et al. Neuroprotective Effects of 7,8-dihydroxyflavone on Midbrain Dopaminergic Neurons in MPP(+)-treated Monkeys. Sci Rep. 2016;6:34339. Available from: https://www.nature.com/articles/srep34339
- Kamarudin MNA, Sarker MMR, Zhou JR, Parhar I. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J Exp Clin Cancer Res. 2019;38(1):491. Available from: https://doi.org/10.1186/s13046-019-1495-2
- Dickie P, Felser J, Eckhaus M, Bryant J, Silver J, Marinos N, et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology. 1991;185(1):109-19. Available from: https://doi.org/10.1016/0042-6822(91)90759-5
- Curreli S, Krishnan S, Reitz M, Lunardi-Iskandar Y, Lafferty MK, Garzino-Demo A, et al. B cell lymphoma in HIV transgenic mice. Retrovirology. 2013;10:92. Available from: https://doi.org/10.1186/1742-4690-10-92
- Jaehne EJ, Chong EMS, Sbisa A, Gillespie B, Hill R, Gogos A, et al. TrkB agonist 7,8-dihydroxyflavone reverses an induced prepulse inhibition deficit selectively in maternal immune activation offspring: implications for schizophrenia. Behav Pharmacol. 2021;32(5):404-12. Available from: https://doi.org/10.1097/fbp.0000000000000632
- Wang N, Liu X, Li XT, Li XX, Ma W, Xu YM, et al. 7,8-Dihydroxyflavone Alleviates Anxiety-Like Behavior Induced by Chronic Alcohol Exposure in Mice Involving Tropomyosin-Related Kinase B in the Amygdala. Mol Neurobiol. 2021;58(1):92-105. Available from: https://doi.org/10.1007/s12035-020-02111-0
- Wang Z, Wang SP, Shao Q, Li PF, Sun Y, Luo LZ, et al. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic Biol Med. 2019;145:187-97. Available from: https://doi.org/10.1016/j.freeradbiomed.2019.09.033
- Garcia-Diaz Barriga G, Giralt A, Anglada-Huguet M, Gaja-Capdevila N, Orlandi JG, Soriano J, et al. 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington's disease mouse model through specific activation of the PLCgamma1 pathway. Hum Mol Genet. 2017;26(16):3144-60. Available from: https://doi.org/10.1093/hmg/ddx198
- Stagni F, Uguagliati B, Emili M, Giacomini A, Bartesaghi R, Guidi S. The flavonoid 7,8-DHF fosters prenatal brain proliferation potency in a mouse model of Down syndrome. Sci Rep. 2021;11(1):6300. Available from: https://doi.org/10.1038/s41598-021-85284-5