More Information
Submitted: September 11, 2023 | Approved: September 26, 2023 | Published: September 27, 2023
How to cite this article: Monti L, Roscio DD, Tutino F, Casseri T, Arrigucci U, et al. Stroke Mimics: Insights from a Retrospective Neuroimaging Study. J Neurosci Neurol Disord. 2023; 7: 094-103.
DOI: 10.29328/journal.jnnd.1001083
Copyright License: © 2023 Monti L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Stroke mimic; Acute ischemic stroke; Arterial spin labeling; Advanced MRI; Brain perfusion
Stroke Mimics: Insights from a Retrospective Neuroimaging Study
Lucia Monti1*, Davide del Roscio1, Francesca Tutino2, Tommaso Casseri1, Umberto Arrigucci1, Matteo Bellini1, Maurizio Acampa3, Sabina Bartalini4, Carla Battisti5, Giovanni Bova6 and Alessandro Rossi1,4,5
1Diagnostic and Functional Neuroimaging Unit, Department of Neurology and Human Movement Sciences, University Hospital of Siena, Italy
2Nuclear Medicine Unit, Sant’ Andrea Hospital, La Spezia, Italy
3Stroke Unit, Department of Emergency-Urgency and Transplants, University Hospital of Siena, Italy
4Neurology Unit, Department of Neurology and Human Movement Sciences, University Hospital of Siena, Italy
5Neurometabolic Unit, Department of Neurology and Human Movement Sciences, University Hospital of Siena, Italy
6Emergency-Urgency Unit, Department of Emergency-Urgency and Transplants, University Hospital of Siena, Italy
*Address for Correspondence: Lucia Monti, MD, Unit of Diagnostic and Functional Neuroimaging, Department of Neurology and Human Movement Sciences, University Hospital of Siena, Santa Maria alle Scotte, Viale Bracci 2, 53100 Siena, Italy, Email: [email protected]; [email protected]
Objectives: The study’s goals are to evaluate the management of Stroke Mimics (SMs), conditions with stroke-like symptoms but non-vascular origins. It seeks to assess the effect of unnecessary intravenous thrombolysis on therapy delays and determine the best SMs diagnosis approach.
Materials and methods: A review was conducted of all patients admitted to the Emergency Department under a “stroke code” from January 1, 2018, to January 31, 2019. Anamnestic and clinical data, along with information on neuroimaging protocols and findings, were collected. Advanced MRI sequences, such as Arterial Spin Labeling (ASL) MR perfusion and MR spectroscopy, were revised to confirm the diagnoses.
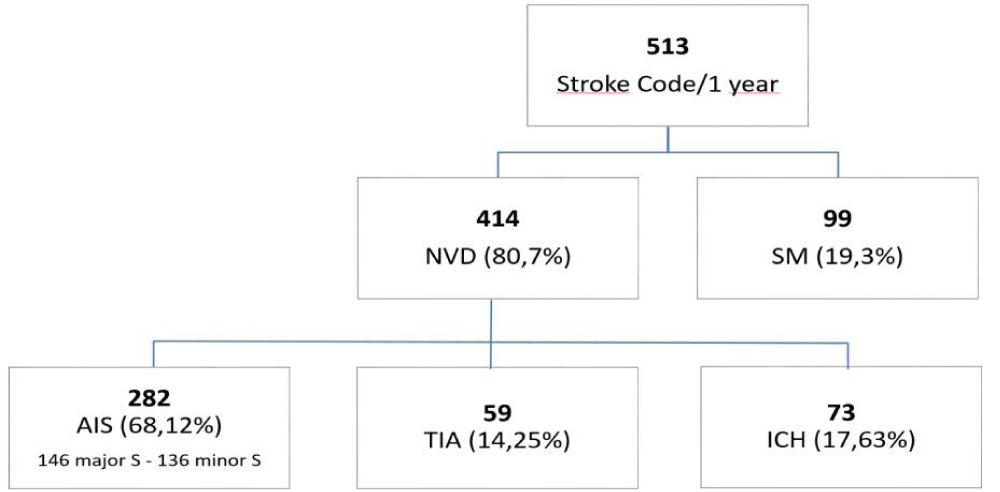
Results: 513 stroke codes were found; a neurovascular disease was diagnosed in 414 cases: 282 ischemic strokes and 73 intracranial hemorrages and 59 TIAs. The 99 SMs included, 13 infections, 12 syncopes, 11 epileptic seizures, 11 hemodinamic conditions, 10 tumors, 9 metabolic disorders, 9 diziness, 7 migraines, 4 drug/alcohol intoxication, 3 functional disorders, 3 acute hydrocefalus, 2 multiple sclerosis, 2 arteriovenous malformations, 1 spinal cord compression and 2 unexplained conditions. Specific neuroimaging findings were evaluated for all cases. Positive and negative predictive values of clinical diagnosis of SM were respectively 0.23 and 0.35. 125 SM patients underwent MRI examination, 40 of which within 3 hours from the activation of stroke code. Advanced MRI sequences as ASL, MR perfusion, MR spectroscopy were used to reach the correct diagnosis.
Conclusions: Advanced multimodal MRI can be a valuable tool in the assessment of, particularly in cases where conventional imaging techniques such as DWI-FLAIR mismatch are inconclusive. The novelty of this retrospective study is to demonstrate that the consistent use of arterial spin labeling perfusion in cases with stroke code leads to a rapid and accurate diagnosis of SMs. The implementation of an MRI-based pathway can expedite the diagnosis and treatment of underlying non-vascular causes such as SMs.
Stroke Mimics (SMs) refer to a wide spectrum of non-vascular/non-ischemic conditions characterized by acute, focal neurological deficits at presentation, simulating ischemic stroke symptoms. SMs correspond to patients diagnosed with acute stroke at the admission to the hospital not receiving a definite diagnosis of stroke during the diagnostic procedures in the acute setting (acute stroke false positives). The main concern about SMs is to avoid unnecessary thrombolysis, not beneficial for these patients that need instead the referral to different clinicians and targeted therapy [1]. However, the complication rate of intravenous thrombolysis in SMs setting was found to be low [2]. SMs represent a significant percentage of all acute stroke hospital admission, ranging up to 30% - 43% in different multicentre studies [3]. Recently a large monocentric, prospective study on 1361 patients based on MRI screening investigated the overall incidence of SMs and the prevalence of different SMs aetiologies in the stroke care pathway [4]. SMs overall prevalence was 38% and the most frequent SMs diagnosis at discharge resulted headache disorders (18.6%), psychological disorder (16.7%), peripheral vertigo (11.9%) and epilepsy (10.6%) [4]. Further studies similarly found that the group of headache disorders and epileptic seizures is the most represented diagnostic category in SMs patients [5]. Other common SMs causes include medical conditions such as sepsis/infections and metabolic disorders, mass lesions, transient global amnesia, mononeuropathy or radiculopathy and demyelinating disease [6]. In addition, several vascular lesions other than stroke can present with stroke-like symptoms such as subdural hematoma, arteriovenous malformations and Reversible Cerebral Vasoconstriction Syndrome, an increasingly recognized entity in children [7]. When clinical scoring system designed for stroke suffers from a lack of specificity, especially in case of absence of cardiovascular risk factors, female sex and young age, neuroimaging, in particular MRI, helps to recognize real stroke and stroke mimics [8]. Neuroimaging, beyond establishing a differential diagnosis between haemorrhagic and ischemic stroke, is able to differentiate cerebrovascular diseases from structural or functional, reversible causes of neurological deficits [9]. Actually, is well established that the identification rate of patients with SMs increases with the use of MRI at baseline respect to CT-based protocols [10,11], due to Diffusion Weighted Imaging (DWI) normal findings [4,12] but also to advanced non-contrast perfusion MRI techniques such as ASL [13]. The advantages of basic MRI scans and DWI in emergency assessment of SMs have been widely outlined in literature, however to our knowledge the role of perfusion weighted MRI in this setting, being suggested in a number of case reports, has not been systematically evaluated yet. This retrospective study aims to highlight, through the presentation of some clinical cases, the advantage of advanced imaging in the early diagnosis of SMs within the wide range of stroke codes. The systematic and harmless study of cerebral perfusion can indeed help identify physiopathological mechanisms underlying non-purely vascular conditions where fibrinolysis should not be employed.
A retrospective chart review was conducted on all cases admitted to the Emergency Unit of Siena University Hospital, a hub center in stroke management, with an acute stroke presentation between January 2018 and January 2019. Written informed consent was obtained from all patients before conducting CT and MRI, following the rules of the Ethics Board of the General Hospital “Santa Maria alle Scotte,” University of Siena, Italy. The University Hospital database was used, and privacy rules were followed to anonymize the files of individual patients.
Only subjects presenting with an acute onset of focal neurological deficits were included. The clinical suspicion of ischemic stroke and therefore the stroke code was activated by emergency physician during the first aid. At emergency department admission, the stroke code was confirmed by neurologist and the patients followed the pathway as shown in the flowchart. The final diagnosis was pulled out from the administrative database of Department of Emergency, Stroke Unit, Neuroscience and Clinical Departments. Patients under the age of 18 were excluded. These presentations, henceforth referred to as SMs, were tabulated, and then compared with the total number of patients correctly admitted with acute ischemic stroke. Patients discharged with the same diagnosis they had on admission were considered the control group and the positive and negative predictive values of clinical diagnosis of SMs and acute stroke were established. The patients were divided in different subgroups such as TIAs (transient ischemic attacks), ischemic strokes, hemorrhagic strokes and SMs. TIA diagnosis was based on regression of the acute focal neurological deficits and concomitant negative imaging (DWI sequence acquired when the clinical assessment was uncertain). All non-ischemic pathologies were classified as SMs. The retrospective analysis was performed on not-contrast-enhanced CT (NCCT) (acquired in all patients), CT Angiography (CTA), CT Perfusion (CTP) and MRI. The neuroimaging protocol was determined by the neuroradiologist and the clinician, based on the clinical scenario. Depending on the situation, the protocol varied. In cases of strong clinical suspicion of acute ischemic stroke, a protocol including CTA and CTP after NCCT was used. Alternatively, if intraparenchymal hemorrhage was detected at NCCT, especially if in an “atypical” location, only CTA was performed. To demonstrate blood-brain permeability alterations, a delayed CT scan after contrast medium administration was performed. If the CT examination proved to be inconclusive or if there was a need to confirm a diagnostic hypothesis, an MRI examination was performed.
The revised MRI examinations were those performed within 21 days from the hospital admission. Susceptibility Weighted Imaging and T1-weighted sequences were revised to assess intracranial haemorrhagic foci and conditions such as cortical hemosiderosis. The first clinical and instrumental evaluation in the emergency department was compared with retrospective evaluation of all imaging findings [non-contrast-enhanced CT, CT Angiography (CTA), CT Perfusion (CTP), MRI] and with the final discharged diagnosis. Considering as controls, the patients with the admission diagnosis equal to discharge diagnosis, diagnostic accuracy as well as positive and negative predictive values were calculated.
CT examination
First intention CT examination was performed with General Electric (Boston, Massachussets, USA) Light Speed VCT 64 using the following protocols: non-contrast brain CT with a slice thickness of 5 mm (120 Kv, 120 - 350 mAs), CTA with slice thickness of 0.6 mm (100 Kv, 100 - 650 mAs), CTP with 5 mm slice thickness (80 kv, 500 mAs, shuttle mode).
MRI examination
MRI scans were performed on a Siemens (Munich, Germany, Europe) Avanto 1.5T scanner with a 12-channel head-coil. A standardized protocol was applied using T1-weighted, T2-weighted, FLAIR and DWI sequences. Pulsed ASL images were acquired using a PICORE Q2T sequence (TR = 3,100 ms, TE = 22 ms, TI1 = 700 ms, TI2 = 1,800 ms, number of slices = 16, thickness = 6.3 mm, gap = 1.6 mm, imaging matrix = 84 ⇥ 84, flip angle [FA] = 90, and acquisition duration = 4 min 45 s). T1-weighted 3D-MPRAGE sequences (TR = 1,880 ms, TE = 3.38 ms, TI = 1,100 ms, FA = 15, number of slices = 176, thickness = 1 mm, gap = 0 mm, and imaging matrix = 256 ⇥ 256) were used to obtain high-resolution T1-weighted axial images covering the whole brain.
Out of the 513 stroke codes recorded within a one-year period, 275 cases were discharged with a diagnosis of ischemic stroke, 171 cases had other causes (including 39 SMs, 59 TIAs, and 73 intraparenchymal, extraparenchymal, and subarachnoid hemorrhages), and 67 cases required further diagnostic evaluation. The initial clinical and instrumental evaluation in the emergency department identified 98 cases (19.1%) as SMs and 275 cases (53.6%) as ischemic strokes. The analysis of the final discharge diagnosis and the retrospective evaluation of CTA, CTP, and MRI examinations revealed 99 cases (19.29%) classified as SMs (excluding 59 TIAs), and 355 cases of neurovascular diseases, including 282 confirmed ischemic stroke events (54.9%) and 73 cases of hemorrhagic strokes (20.79%). Among the 282 confirmed ischemic strokes, 146 (51.77%) were classified as major stroke events, and 136 (48.22%) were minor strokes (Table 1). Among the 99 SMs, the identified pathologies were as follows: 13 cases of infection (sepsis, meningitis, encephalitis) (13.1), 12 cases of syncope (12.1%), 11 cases of epilepsy (11.1%), 11 cases of haemodynamic conditions (pulmonary embolisms, heart failure, etc.) (11.1%), 10 cases of tumors (glioblastoma, meningioma, metastasis, paraneoplastic syndrome) (10.1%), 9 cases of metabolic disorders (electrolytic and glycemic disorders) (9.1%), 9 cases of dizziness (peripheral vestibular disorders and peripheral neuropathy) (9.1%), 7 cases of migraine (7.1%), 4 cases of exogenous toxins (drugs and/or alcohol) (4%), 3 cases of functional disorders (3%), 3 cases of acute hydrocephalus (3%), 2 cases of multiple sclerosis (2%), 2 cases of arteriovenous malformation (2%), 1 case of spinal cord compression (1%), and 2 cases of unexplained conditions (2%) (Table 2). The diagnostic accuracy of the clinical diagnosis of SMs was 18.5%, with positive and negative predictive values of 0.23 and 0.35, respectively. In terms of ischemic stroke diagnosis, no false negatives were observed, indicating the absence of stroke chameleons in our population. Additionally, within 21 days of hospital admission, a total of 125 subjects (24.2% of all patients) underwent MRI examinations, with 40 of those performed within 3 hours from the activation of the stroke code. Advanced MRI sequences such as ASL, MR perfusion, and MR spectroscopy were utilized to reach an accurate diagnosis. Upon admission, all subjects underwent non-contrast-enhanced CT (NCCT) scans (513/513). Out of these, 136 subjects were assessed with NCCT scans only (26.5%). A total of 372 subjects underwent concurrent CTA scans at admission (72.5%), and 223 subjects were evaluated with both CTA and CTP scans (NCCT + CTA + CTP) (43.5%). Four subjects received a delayed scan after the administration of iodine contrast medium (less than 1%). One subject was excluded because deceased before imaging acquisition (less than 1%).
| Table 1: Data of patients admitted as suspected stroke and different characteristics of subgroups. | |||||
| TOT | AIS | ICH | SM | TIA | |
| N | 513 | 282 | 73 | 99 | 59 |
| Male / Female (n) | 286 / 227 | 145 / 137 | 43 / 30 | 69 / 30 | 33 / 26 |
| Age (years old, mean ± s.d.) | 73,5 (+14,37) | 76,34 (+12,20) | 73,86 (+12,07) | 67,02 (+17,12) | 76,65 (+12,94) |
| Baseline NIHSS (median, IQR) | 2 (0 – 8) | 8 (3 – 12) | 6,5 (3 – 9) | 1 (0 – 2) | 0 (0 -1,75) |
| N. of patients who underwent multimodal imaging (CT and MRI) | 125 (24,37%) | 35 (12,41%) | 17 (23,29%) | 29 (29,29%) | 44 (74,58%) |
| Patients-reported outcomes concordance between first aid and ED discharge diagnosis was 49.12% (252/513), while between ED discharge diagnosis and hospital discharge letter was 83.4% (428/513). AIS = Acute Ischemic Stroke; ICH = Intracranial Hemorrage; SM = Stroke Mimic; TIA = Transient Ischemic Attack. |
|||||
| Table 2: Prevalence of different discharge diagnosis in our SMs patients and TIAs compared with literature reports. n.a.: not applied. † In the present study haemorrhages are not included as SMs. Haemorrhages are here reported for completeness and comparison with literature data. | |||
| Discharge diagnosis | University Hospital of Siena (12 months) % (n) |
St George’s Hospital, London5 (14 months) % (n) |
Strasbourg University Hospital4 (12 months) % (n) |
| Epilepsy | 11.1% (11) | 12% (66) | 10,6% (55) |
| Peripheral vertigo | 9.1% (9) | 6.8% (5) | 11.9% (62) |
| Syncope | 12.1% (12) | 7% (39) | n.a. |
| Headache disorders | 7.1% (7) | 15% (85) | 18.6% (97) |
| Sepsis | 13.1% (13) | 3.7% (21) | 1.5% (8) |
| Haemorrhages | 31.6%† (73) | n.a. | 2.7% (14) |
| Brain tumor | 10.2% (10) | 2.7% (15) | 2.1% (11) |
| Functional disorders | 3% (3) | 6.4% (36) | 16.7% (86) |
| Acute Hydrocephalus | 3% (3) | n.a. | 3.6% (19) |
| Metabolic | 9.1% (9) | n.a. | 2.1% (11) |
| Arteriovenous malformation | 2% (2) | n.a. | n.a. |
| Drug/alcohol toxicity | 4% (4) | 2.2% (12) | 3.4% (18) |
| Multiple sclerosis | 2% (2) | n.a. | n.a. |
| Unexplained | 2% (2) | 10% (60) | n.a. |
| Haemodynamic conditions | 11.1% (11) | n.a | n.a. |
| Spinal cord compression | 1% (1) | n.a. | n.a |
| TIA (*) | 11.5% (59) | n.a | 42.5% (236) |
| Total (n) | 231 | 521 | 555 |
| Quenardelle, et al. [4]; Dawson, et al. [5]. (*) Patients with TIA were considered when imaging showed no evidence of ischemic lesions, and the discharge diagnosis ruled out vascular events. | |||
The found prevalence of SMs, at 19.29% among all stroke admissions, is slightly lower than what is reported in the literature. Specifically, our rate of SMs is lower than the 38% value found in a prospective study by Quenardelle, et al. [4] that used MRI screening for stroke code patients, as well as in a retrospective study by Liberman, et al. [3].
Since in our study, only 32% of SM patients underwent MRI scans within the first 3 hours from the activation of the stroke code, the incidence of SMs might have been higher if MR examinations had been performed for all stroke codes [13]. Liberman, et al. [14] also suggested that the high prevalence of SMs could be related to the significant reduction in door-to-needle time, which could lead to increased errors due to less time available for diagnostic formulation when not using the CT approach exclusively.
In our case series, the door-to-needle time optimization might contribute to the extremely low (18.5%) diagnostic accuracy, as well as the negative and positive predictive values (respectively 0.35 and 0.23) for the clinical SMs diagnosis, confirming the difficulty to differentiate clinically an ischemic stroke from other diagnosis in patients presenting with acute neurological deficits. The imperfect accuracy and the small negative predictive value of clinical assessment of SMs in our patient population underline the value of MRI implementation in emergency protocols.
MRI advantages over CT-based protocols in SMs management are related to better diagnostic accuracy for recent ischemia due to DWI sequences, to improved detectability of posterior fossa lesions, lacunar infarction and brain tumors, as well as inflammatory and metabolic disorders not evident on CT examination. Past prospective studies showed that the 38% - 43% SMs incidence at initial clinical and laboratory evaluation declines to 4% - 6.5% with CT scanning at admission [15] and drops to further 0% - 1.3% with MRI imaging in addition [11,16]. Moreover, an early MRI diagnosis could impact both SMs and acute ischemic strokes management in terms of length of stay and imaging resources within the health service. In this single centre experience, MRI examination was performed when the diagnosis was not really clear (24,6%) with a mean delayed diagnosis time of 4,6 days. Knowing that the waste of time is harmful in all patients, actually the main concern is that if patients are not correctly identified, therefore they cannot be properly treated. CT, CTA, CTP and standard MRI acquisitions could not be able to improve the diagnostic accuracy of SM. Quantitative technique such as MR ASL perfusion offers the possibility to evaluate functional, hemodynamic parameters not explored by conventional imaging. Cerebral Blood Flow (CBF) map, beyond demonstrating ischemic penumbra in stroke, displays typical patterns with peculiar features respect to real stroke (especially when coupled with DWI) in different SM such as epileptic seizures, in particular post-ictal paresis [17-19] (Figures 1,2), brain tumors [20,21] (Figure 3) arteriovenous malformations or fistulas (Figure 4) migraine with aura, other primary headache disorders [22,23], inflammatory or infectious conditions like multiple sclerosis (Figure 5) or encephalitis (Figure 6), metabolic disorder like hypoglycaemic hemiparesis [24,25], or other unusual causes (Figure 7). ASL perfusion, a contrast medium-free MRI perfusion technique, appears more suitable than bolus methods in an emergency care setting where creatinine blood level may not be readily available or contrast medium may be contraindicated [26]. In this technique blood acts as an “endogenous tracer” and is able to quantify the CBF [27,28]. The latter propriety is unique in comparison with other imaging acquisition. Moreover, CTP, is usually acquired on a limited section of brain tissue after bolus administration of contrast medium, while perfusion weighted MRI by using ASL sequences brings the benefits of whole brain acquisition besides the opportunity to avoid contrast medium administration. It’s well known that non-contrast CT and CTA negativity does not exclude stroke and only few MRI sequences as diffusion restriction highly sensitive for acute ischemia cannot help in cases of diagnostic uncertainty and increase the incidence of SMs. Multimodal MRI applied in the stroke care pathway as the first line imaging could increase the diagnostic accuracy of acute neurological deficit without loss of time and lead to correct patient management. The main limitation of the present study consists of its retrospective nature; however, this analysis can help to adjust the current diagnostic approach and to draw the way of future prospective studies regarding the best SMs management.
Figure 1: Final discharged diagnosis: neurovascular diseases (NVD); acute ischemic stroke (AIS); intracranial haemorragies (ICH); transient ischemic attack (TIA); ischemic stroke (S).
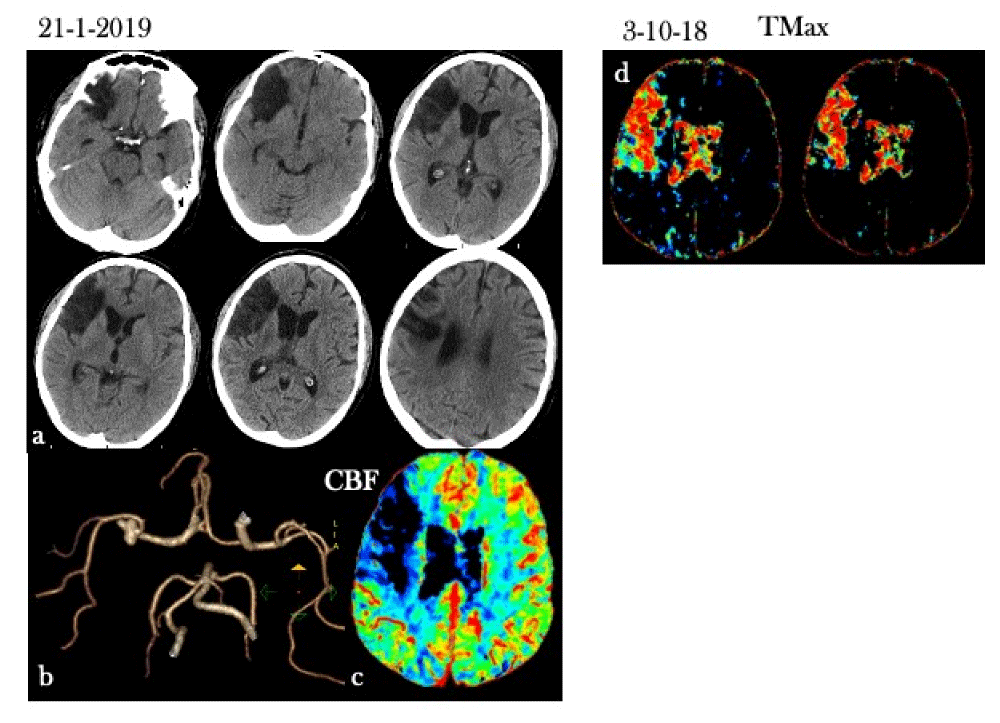
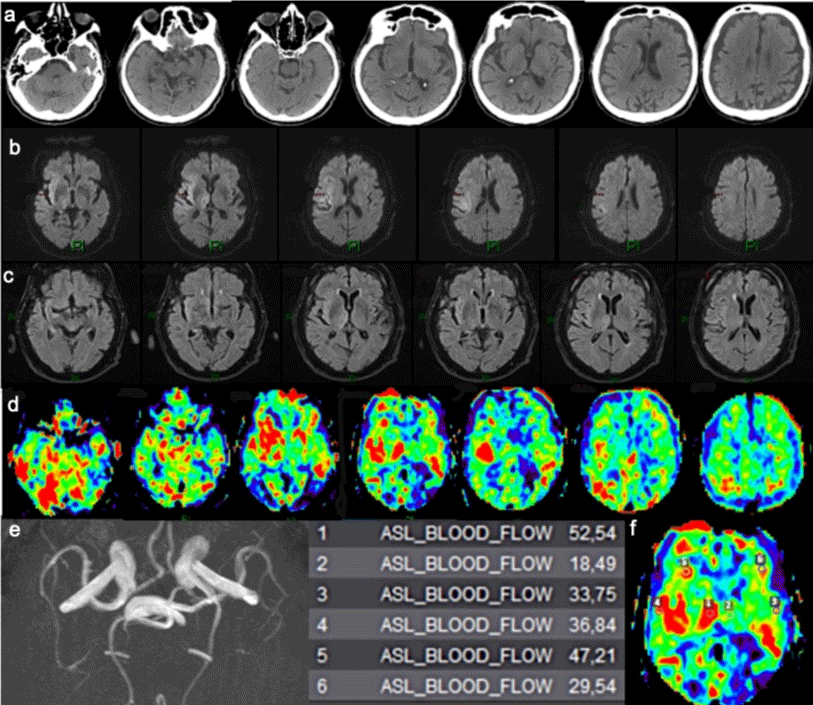
Figure 2: CT perfusion and Todd’s paresis or postictal paresis/paralysis. Stroke code: 62 years old male with symptoms onset as dysarthria, stomachache and syncope, followed by left upper limbs hemiplegia and lower limbs hemiparesis. Clinical History: old right hemisphere ischemic event, no seizure history. a) No-contrast CT: right temporal encephalomalacia as result of old ischemic event. b) CT-angiography did not demonstrate arterial occlusion. c) CT perfusion map CBF: cortico-subcortical hypoperfusion pattern in the right CMA vascular distribution. d) Previous CT perfusion map Tmax (during old stroke: 4 months after): see the Tmax map suggesting a large core on the right CMA vascular territory.
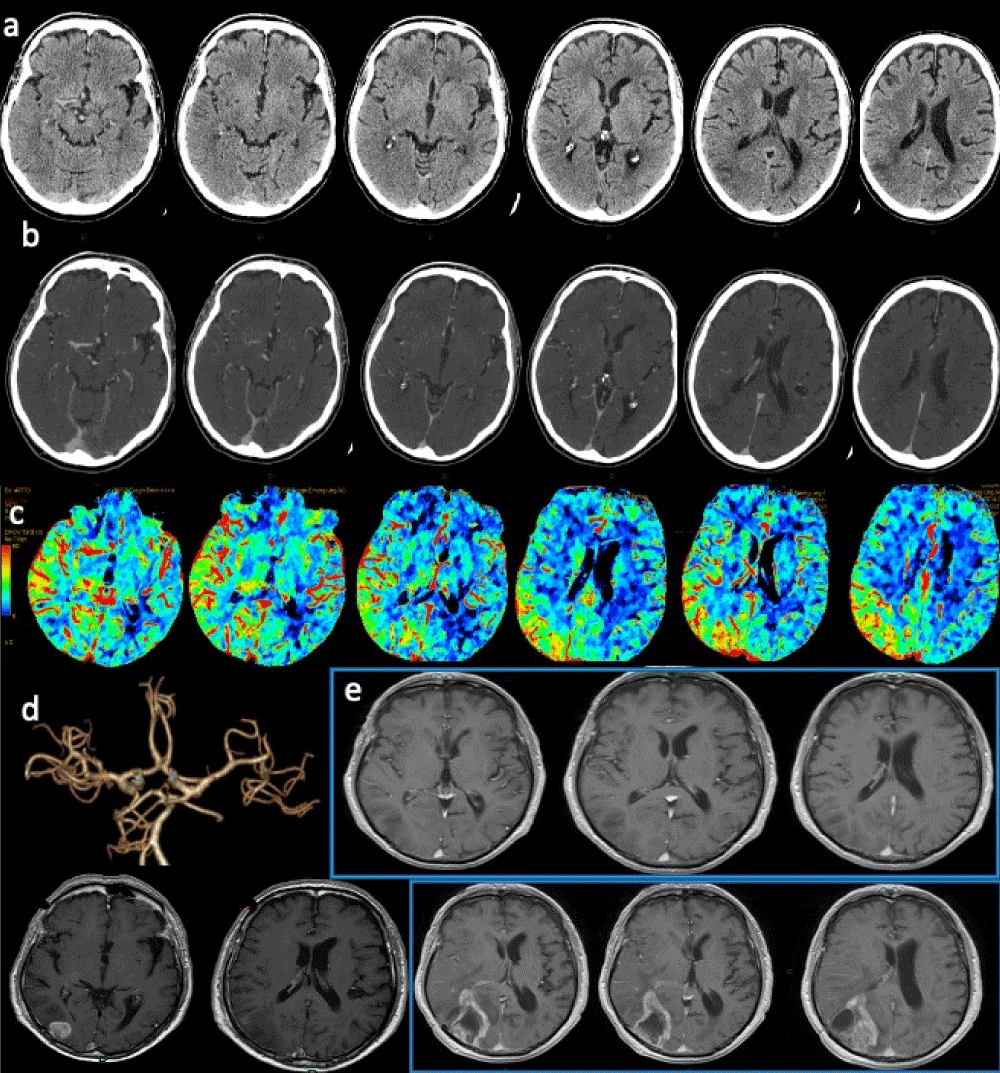
Figure 3: Brain tumor. Stroke code: 74 years old male, presenting with sudden onset of left hemiparesis. a) No-contrast CT: cortical-subcortical hypodensity in the right inferior temporal gyrus and inferior occipital gyrus suggesting edema in the right posterior temporal lobe with mild mass effect on the right ventricular system; b)Contrast medium CT: no evidence of pathological parenchymal enhancement; c) CTP (CBF map) shows large hyperperfusion involving the right parietal-temporo-occipital lobes and d)CTA does not demonstrate any arterial occlusion. e) Contrast enhanced T1 MRI images demonstrate inhomogeneous hypointensity of right inferior temporal gyrus and inferior occipital gyrus without BEE leakage. Follow up scans (contrast-enhanced T1 MRI): d) enhancing lesion in the right inferior temporal gyrus and inferior occipital gyrus. Histology demonstrated high grade glioblastoma. e) Post-surgical T1 contrast images show wide surgical cavity surrounded by markedly irregular enhancing ring of pathological tissue.
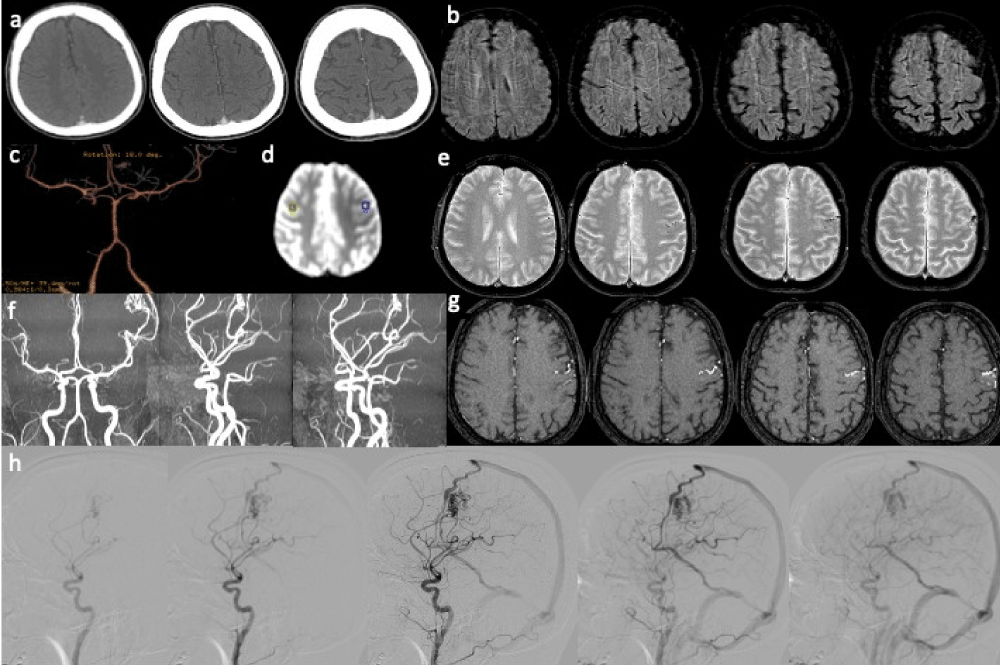
Figure 4: Arteriovenous malformation. Acute focal neurologic symptoms. Stroke code: 45 years old male presenting with right hemiparesis, weakness, impairment of speech and alteration of consciousness (NIHSS 6). a) No-contrast brain CT and b) CTA findings were negative. d) T2- weighted MRI shows ectasic surface vessels in the left frontal lobe, better appreciated on (e) SWI. f) Quantitative ASL (OLEA sphere 3.0): focal hypoperfusion corresponding to the left Rolandic cortical area adjacent to ectasic cortical vessels. 3D-TOF MRA(g,h) (single slice and MIP) shows the abnormal dilated vessels embedded left frontal lobe and (f) the DSA on lateral left carotid angiograms demonstrate the left frontal AVM with a nidus supplied by an enlarged branch of MCA. Note the early drainage to a frontal cortical vein into the superior sagittal sinus (and via an internal cerebral vein to the straight sinus). Diagnosis: frontal AVM inducing focal hypoperfusion of left Rolandic cortical area caused the acute neurologic symptoms.
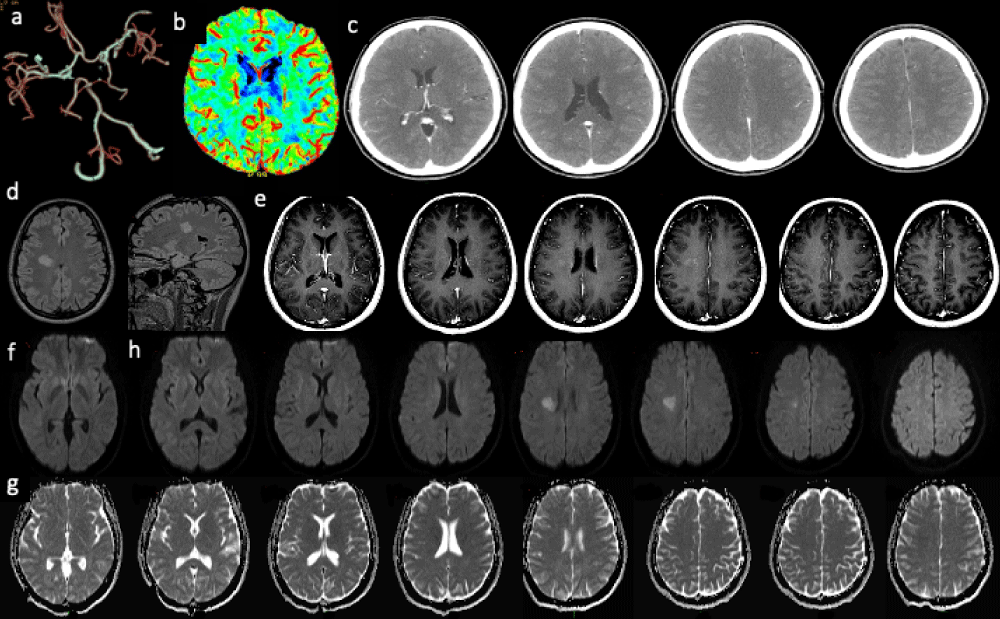
Figure 5: Multiple Sclerosis. Stroke code: 35 years old female presenting with improving left upper limb paresis. In anamnesis: smoker, oral contraceptive therapy. a) CTA: no arterial occlusions were demonstrated b) CTP did not show asymmetric hypoperfusion and c) No acute or chronic intracranial pathology was present on parenchymal phase. Nevertheless, previous informed consens and the absence of contraindication for e.v. fibrinolysis, at about 2.10 h clinical onset: 52 mg /Kg Actilyse was administered. d) A delayed 3D FLAIR MRI demonstrated an oval shaped hyperintensity greater than 2 cm in the right frontal periventricular white matter, with main axis perpendicular to lateral ventricle. f) Artifactual FLAIR/DWI mismatch: corresponding hyperintesity on DWI images not due to low ADC (T2-shine through effect) g), suggesting vasogenic edema. e) Contrast enhanced T1 MRI: heterogeneous and venular enhancement. Diagnosis: Tumor-like multiple sclerosis.
Figure 6: Herpes Simplex Virus Encephalitis. Stroke code: 75 years old female with left hemiparesis and alteration of consciousness. a) No-contrast CT shows loss of grey-white differentiation in the right insula (loss of “ribbon sign”) and lateral temporal lobe. b) DWI MRI: corresponding hyperintensity along the right temporo-insular cortex representing cytotoxic edema. c) T2 FLAIR MRI: cortical hyperintensity and swelling in the right insula, anterolateral temporal lobe and temporo-parieto-occipital junction. d) ASL: Focally increased CBF areas corresponding to right insula, antero-lateral temporal lobe and temporo-occipital junction suggested the right diagnosis: Herpes Simplex Virus Encephalitis (HSVE). e) MR-Angiography confirm no arterial obstruction. The quantitative comparison between the right and left hemisphere is showed in the table of ROI f) and in the corresponding ASL perfusion map.
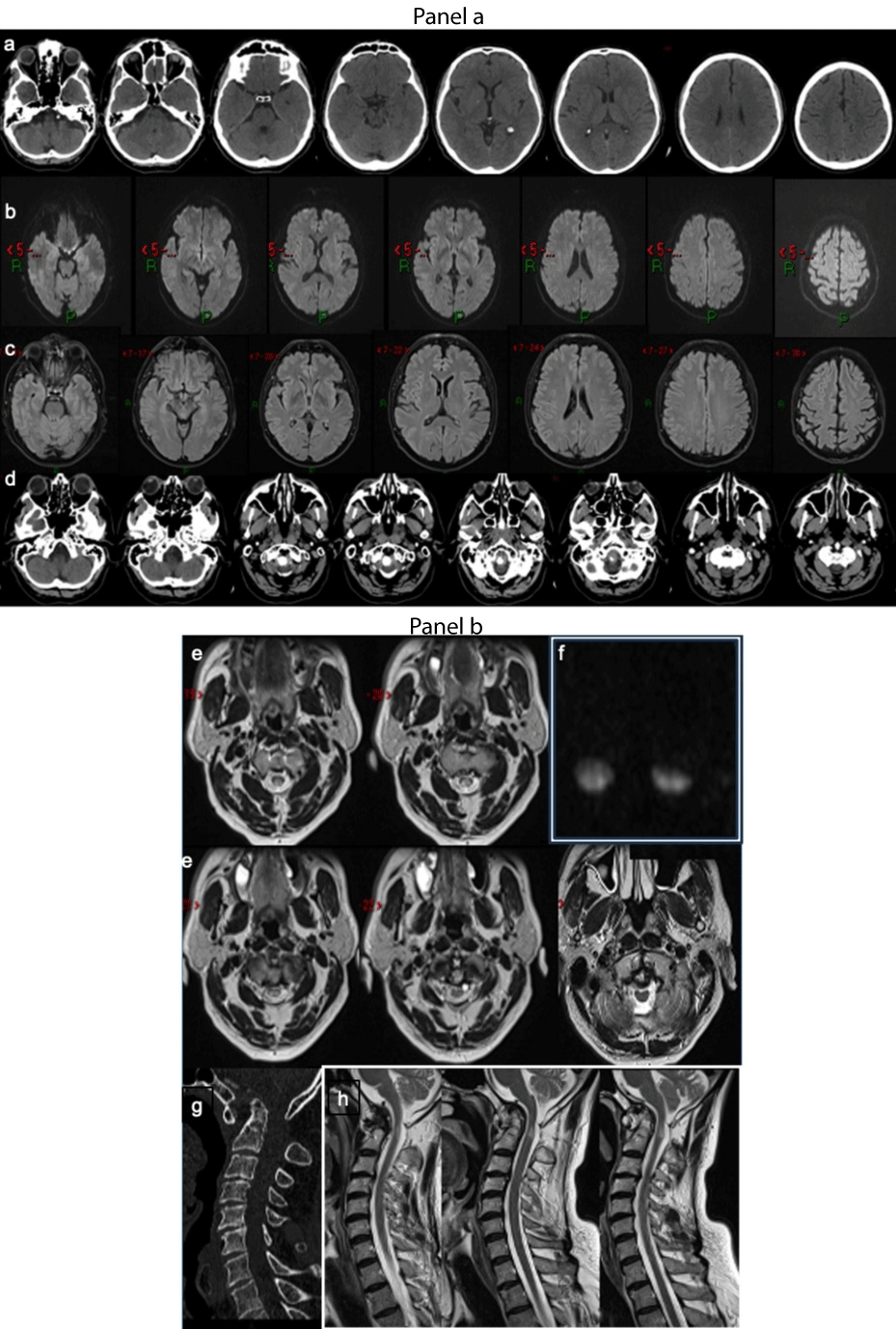
Figure 7: Spinal cord compression. Panel a. Stroke code: 58 years old male presenting with suddenly augmentation right upper limb paresis and paraesthesia. (a) No-contrast brain CT and intracranial CTA (not showed) did not reveal any vascular or parenchymal abnormalities or subacute ischemic lesions MRI did not demonstrate brain areas of restricted diffusion were evident on (b) DWI or FLAIR (c) signal alteration. The non-contrast CT of the cranio-cervical tract shows the odontoid process dislocated superiorly with involvement of the foramen magnum.(d) Panel b. Axial T2-weighted MRI images show “owl-eyes” sign at C1/C2 level (e). Intramedullary lesions at C1/C2 level were confirmed diffusion images (f).Cervical spine CT and sagittal reconstruction (g) and T2 sagittal images revealed an odontoid process subluxation and the focal hyperintensity of cervical spinal cord (h) Diagnosis: Cervical myelopathy. Atlantoaxial instability for degenerative changes caused the spinal cord compression and acute myelopathy.
Appropriate management of ischemic stroke is provided by standard MRI. In fact, DWI and FLAIR sequences are able to assess TIA versus ischemic lesions, to suggest the timing of the ischemic lesion and to define the ischemic lesion load. On the other hand, the majority of pathologies mimicking ischemic stroke could be demonstrated only by improving the diagnostic protocol with advanced sequences such as ASL perfusion. To differentiate ischemic stroke from SMs few hours after the onset of neurological symptoms, which is actually a challenge for both neurologists and radiologists, a multimodal MRI approach may demonstrate early signs of vascular impairment reflecting functional hemodynamic changes without causing ischemic lesions.
Contribution to the field statement
The prevalence of SMs in this study is consistent with previous literature, although it is lower than in some MRI-based studies. The authors suggest that performing MR examinations in all stroke cases could potentially increase the incidence of SMs. The optimization of door-to-needle time contributes to the low diagnostic accuracy of clinical SMs diagnosis, underscoring the value of implementing MRI in emergency protocols. MRI examination in cases of unclear diagnosis and the utilization of quantitative techniques like MR ASL perfusion can evaluate functional and hemodynamic parameters not explored by conventional imaging. ASL perfusion, a contrast medium-free MRI technique, offers the ability to quantify Cerebral Blood Flow (CBF) and can differentiate various SMs from real stroke based on distinct perfusion patterns. Distinguishing ischemic stroke from SMs shortly after the onset of symptoms is a challenge, but a multimodal MRI approach can reveal early signs of vascular impairment without causing ischemic lesions.
Highlights
• Approximately 30% of sudden neurological deficits are attributed to non-ischemic causes.
• The “time is brain” principle overlooks stroke mimic events.
• Multimodal MRI facilitates optimal management of stroke mimics.
- Anathhanam S, Hassan A. Mimics and chameleons in stroke. Clin Med (Lond). 2017 Apr;17(2):156-160. doi: 10.7861/clinmedicine.17-2-156. PMID: 28365629; PMCID: PMC6297626.
- Zinkstok SM, Engelter ST, Gensicke H, Lyrer PA, Ringleb PA, Artto V, Putaala J, Haapaniemi E, Tatlisumak T, Chen Y, Leys D, Sarikaya H, Michel P, Odier C, Berrouschot J, Arnold M, Heldner MR, Zini A, Fioravanti V, Padjen V, Beslac-Bumbasirevic L, Pezzini A, Roos YB, Nederkoorn PJ. Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke. 2013 Apr;44(4):1080-4. doi: 10.1161/STROKEAHA.111.000126. Epub 2013 Feb 26. PMID: 23444310.
- Liberman AL, Prabhakaran S. Stroke Chameleons and Stroke Mimics in the Emergency Department. Curr Neurol Neurosci Rep. 2017 Feb;17(2):15. doi: 10.1007/s11910-017-0727-0. PMID: 28229398.
- Quenardelle V, Lauer-Ober V, Zinchenko I, Bataillard M, Rouyer O, Beaujeux R, Pop R, Meyer N, Delplancq H, Kremer S, Marescaux C, Gény B, Wolff V. Stroke Mimics in a Stroke Care Pathway Based on MRI Screening. Cerebrovasc Dis. 2016;42(3-4):205-12. doi: 10.1159/000445956. Epub 2016 Apr 26. PMID: 27111336.
- Dawson A, Cloud GC, Pereira AC, Moynihan BJ. Stroke mimic diagnoses presenting to a hyperacute stroke unit. Clin Med (Lond). 2016 Oct;16(5):423-426. doi: 10.7861/clinmedicine.16-5-423. PMID: 27697802; PMCID: PMC6297311.
- Nau KC, Crocco T, Biola J, Larrabee H. Is it stroke, or something else? J Fam Pract. 2010 Jan;59(1):26-31. PMID: 20074498.
- Lehman LL, Danehy AR, Trenor CC 3rd, Calahan CF, Bernson-Leung ME, Robertson RL, Rivkin MJ. Transient Focal Neurologic Symptoms Correspond to Regional Cerebral Hypoperfusion by MRI: A Stroke Mimic in Children. AJNR Am J Neuroradiol. 2017 Nov;38(11):2199-2202. doi: 10.3174/ajnr.A5296. Epub 2017 Jul 13. PMID: 28705823; PMCID: PMC7963593.
- Allder SJ, Moody AR, Martel AL, Morgan PS, Delay GS, Gladman JR, Fentem P, Lennox GG. Limitations of clinical diagnosis in acute stroke. Lancet. 1999 Oct 30;354(9189):1523. doi: 10.1016/s0140-6736(99)04360-3. PMID: 10551501.
- Mair G, Wardlaw JM. Imaging of acute stroke prior to treatment: current practice and evolving techniques. Br J Radiol. 2014 Aug;87(1040):20140216. doi: 10.1259/bjr.20140216. Epub 2014 Jun 17. PMID: 24936980; PMCID: PMC4112390.
- Hodler J, Kubik-Huch RA, von Schulthess GK, editors. Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [Internet]. Cham (CH): Springer; 2020. PMID: 32119229.
- Moulin S, Leys D. Stroke mimics and chameleons. Curr Opin Neurol. 2019 Feb;32(1):54-59. doi: 10.1097/WCO.0000000000000620. PMID: 30239360.
- Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, Gonzalez RG, Yamada K, Sorensen GA, Koroshetz WJ. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999 Jun 10;52(9):1784-92. doi: 10.1212/wnl.52.9.1784. PMID: 10371524.
- Vilela P. Acute stroke differential diagnosis: Stroke mimics. Eur J Radiol. 2017 Nov;96:133-144. doi: 10.1016/j.ejrad.2017.05.008. Epub 2017 May 5. PMID: 28551302.
- Liberman AL, Liotta EM, Caprio FZ, Ruff I, Maas MB, Bernstein RA, Khare R, Bergman D, Prabhakaran S. Do efforts to decrease door-to-needle time risk increasing stroke mimic treatment rates? Neurol Clin Pract. 2015 Jun;5(3):247-252. doi: 10.1212/CPJ.0000000000000122. PMID: 26124982; PMCID: PMC4469347.
- Kothari RU, Brott T, Broderick JP, Hamilton CA. Emergency physicians. Accuracy in the diagnosis of stroke. Stroke. 1995 Dec;26(12):2238-41. doi: 10.1161/01.str.26.12.2238. PMID: 7491643.
- Huff JS. Stroke mimics and chameleons. Emerg Med Clin North Am. 2002 Aug;20(3):583-95. doi: 10.1016/s0733-8627(02)00012-3. PMID: 12379962.
- Mathews MS, Smith WS, Wintermark M, Dillon WP, Binder DK. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd's paresis. Neuroradiology. 2008 May;50(5):397-401. doi: 10.1007/s00234-008-0362-1. PMID: 18278489.
- Rupprecht S, Schwab M, Fitzek C, Witte OW, Terborg C, Hagemann G. Hemispheric hypoperfusion in postictal paresis mimics early brain ischemia. Epilepsy Res. 2010 May;89(2-3):355-9. doi: 10.1016/j.eplepsyres.2010.02.009. Epub 2010 Mar 16. PMID: 20236802.
- Van Cauwenberge MGA, Dekeyzer S, Nikoubashman O, Dafotakis M, Wiesmann M. Can perfusion CT unmask postictal stroke mimics? A case-control study of 133 patients. Neurology. 2018 Nov 13;91(20):e1918-e1927. doi: 10.1212/WNL.0000000000006501. Epub 2018 Oct 17. PMID: 30333164.
- Morgenstern LB, Frankowski RF. Brain tumor masquerading as stroke. J Neurooncol. 1999 Aug;44(1):47-52. doi: 10.1023/a:1006237421731. PMID: 10582668.
- Krishnaiah B, Ermak D. Anaplastic Astrocytoma Presenting as Ischemic Stroke: A Diagnostic Pitfall. Austin J Cerebrovasc Dis & Stroke. 2017; 4:1058.
- Friberg L, Olsen TS, Roland PE, Lassen NA. Focal ischaemia caused by instability of cerebrovascular tone during attacks of hemiplegic migraine. A regional cerebral blood flow study. Brain. 1987 Aug;110 (Pt 4):917-34. doi: 10.1093/brain/110.4.917. PMID: 3651801.
- Lindahl AJ, Allder S, Jefferson D, Allder S, Moody A, Martel A. Prolonged hemiplegic migraine associated with unilateral hyperperfusion on perfusion weighted magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002 Aug;73(2):202-3. doi: 10.1136/jnnp.73.2.202. PMID: 12122185; PMCID: PMC1737998.
- Sugita Y, Koyanagi M, Oda M, Kobayashi T, Narumi O, Saiki M. Severe Hypoglycemia-induced Right Hemiparesis with Reversible Diffusion Restriction in the Left Internal Capsule Due to Combination Therapy Using Disopyramide and Clarithromycin. NMC Case Rep J. 2017 Nov 21;5(1):31-33. doi: 10.2176/nmccrj.cr.2017-0045. PMID: 29354336; PMCID: PMC5767484.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci. 2012 Aug;117(3):347-51. doi: 10.3109/03009734.2011.652748. Epub 2012 Jan 17. PMID: 22247979; PMCID: PMC3410296.
- Morello A, Casseri T, Acampa M, Galluzzi P, Cerase A, Monti L. Stroke in Pregnancy and Review of Current Literature: Arterial Spin-Labeling MRI Can Identify the Presence and Intensity of Collateral Circle. J Stroke Cerebrovasc Dis. 2018 Dec;27(12):3575-3577. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.027. Epub 2018 Sep 15. PMID: 30228010.
- Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology. 2016 Nov;281(2):337-356. doi: 10.1148/radiol.2016150789. PMID: 27755938.
- Ferré JC, Bannier E, Raoult H, Mineur G, Carsin-Nicol B, Gauvrit JY. Arterial spin labeling (ASL) perfusion: techniques and clinical use. Diagn Interv Imaging. 2013 Dec;94(12):1211-23. doi: 10.1016/j.diii.2013.06.010. Epub 2013 Jul 11. PMID: 23850321.