More Information
Submitted: June 12, 2021 | Approved: June 28, 2021 | Published: June 29, 2021
How to cite this article: Cirio JJ, Ciardi C, Lopez M, Scrivano EV, Lundquist J, et al. Endovascular management of tandem occlusions in stroke: Treatment strategies in a real-world scenario. J Neurosci Neurol Disord. 2021; 5: 055-060.
DOI: 10.29328/journal.jnnd.1001051
ORCiD: orcid.org/0000-0001-9843-5544
Copyright License: © 2021 Cirio JJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Acute ischemic stroke; Tandem occlusion; Large vessel occlusion; Endovascular
Endovascular management of tandem occlusions in stroke: Treatment strategies in a real-world scenario
Juan J Cirio1*, Celina Ciardi1, Matias Lopez1, Esteban V Scrivano2, Javier Lundquist2, Ivan Lylyk2, Nicolas Perez2 and Pedro Lylyk2
1Department of Interventional Neuroradiology. Instituto Medico ENERI, Clinica La Sagrada Familia, Buenos Aires, Argentina
2Department of Vascular Neurology, Stroke Unit. Instituto Medico ENERI, Clinica La Sagrada Familia, Buenos Aires, Argentina
*Address for Correspondence: Dr. Juan J Cirio, Instituto Medico Eneri Av Libertador 6647, Buenos Aires, 1430 Argentina, Tel: +54 9 11 4406 9283; Email: [email protected]
The association between intracranial large vessel occlusion (LVO) and concurrent steno-occlusive lesion of an ipsilateral extracranial internal carotid artery (ICA) is considered a tandem occlusion (TO) [1]. In approximately half of TO, the first clinical manifestation are acute occlusions of the extracranial ICA associated with occlusion of the middle cerebral artery (MCA), with additional occlusion of the intracranial ICA in up to 25% of these cases.[2] This particular lesion subset is technically challenging for endovascular treatment (EVT) and is also characterized by lower success rates of intravenous thrombolysis [3], worse prognosis compared to intracranial occlusions alone, and higher rates of symptomatic intracranial hemorrhage [4]. The optimal approach regarding EVT of TO remains controversial, and reports in this regard are scarce. There are two proposed strategies according to the selection of the first lesion to be treated. The proximal approach comprises stenting of the proximal cervical ICA followed by mechanical thrombectomy (MT) of the intracranial vessel, whereas the distal approach involves MT followed by stenting of the cervical ICA [3–14].
Besides, there other clinically relevant unresolved aspects regarding the treatment of these patients, such as concomitant use of intravenous thrombolysis, the need for stenting compared to angioplasty alone, as well as the most adequate antiplatelet strategy after treatment. Accordingly, we aimed to report the procedural and clinical outcomes of a real-world experience in a comprehensive stroke center regarding EVT of anterior circulation acute ischemic stroke (AIS) associated with a TO.
Study design and population
We conducted a retrospective evaluation of all patients who underwent EVT for an AIS in a comprehensive stroke center between January 2015 and October 2019 to identify those diagnosed with anterior circulation AIS secondary to TO who received combined extracranial and intracranial EVT. All procedures were performed following the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Written informed consent was obtained from all patients or relatives before the procedure.
Data were retrieved from all patients aged > 18 years who complied with the following: anterior circulation AIS demonstrated on magnetic resonance imaging (MRI) or computed tomography (CT); who received EVT up to 24 hours from the onset of the symptoms or last known to be well (LKW); demonstrated tandem lesions on digital subtraction angiography, CT angiography or MRI angiography; extracranial ICA lesions (regardless of atherosclerotic or dissecting etiology) defined as complete occlusion or stenosis > 90% according to NASCET criteria [15], ipsilateral proximal intracranial occlusions affecting the intracranial ICA or proximal segments of MCA; and combined EVT, consisting of either proximal-first or distal-first strategies, including proximal balloon angioplasty with or without emergent stenting and distal MT using a second-generation stent-retriever device with or without concomitant contact aspiration. Patients were included irrespective of the antiplatelet therapy and intravenous thrombolysis strategies. Patients with signs of intracranial hemorrhage (ICH) demonstrated on CT or SWI-MRI were excluded from the analysis.
Data analysis
Using electronic health records, data regarding demographical characteristics, the baseline “National Institutes of Health Stroke Scale” NIHSS score, baseline diffusion-weighted imaging volume (DWIvol), time metrics, periprocedural antiplatelet therapy, intravenous thrombolysis, and modified Rankin score (mRS) at 90-days after treatment were recorded and analyzed. Alberta Stroke Program Early CT Scores (ASPECTS) were determined using non-contrast CT [16] or DWI-MRI [17] The degree of extracranial stenosis was defined according to the NASCET criteria [15]. DWIvol was measured before the procedure, using a semi-quantitative method [18]. Final reperfusion was classified according to the modified thrombolysis in cerebral infarction (mTICI) grade [19], on the last angiographic image; mTICI grades 2b/3 were considered as successful reperfusion. The occurrence of ICH was diagnosticated with CT or MRI; being symptomatic ICH (sICH) defined according to the European Cooperative Acute Stroke Study (ECASS)-3 definition [20].
Statistical analysis
Continuous variables were expressed as means ± standard deviation; or median, interquartile range (IQR) in case of non-Gaussian distribution. Categorical variables were reported as counts and percentages. Comparisons between groups were performed using independent samples Student’s t-test for continuous variables, and Chi-square or Fisher’s exact test (for small-group analysis) for categorical variables. Non-parametric comparisons were performed using Kruskal-Wallis tests. All analyses were performed using SPSS software (23.0.0.0 SPSS Inc, Chicago, IL), and a p - value < 0.05 indicated significant differences.
Demographic data and clinical characteristics
From January 2015 to October 2019, 268 patients underwent EVT for an AIS with LVO in the anterior territory. Of these, 40 (14.9%) strokes were associated with TO and fulfilled the inclusion criteria.
Demographic data and cardiovascular risk factors are shown in table 1. Baseline NIHSS, radiological data, therapeutic time metrics, and EVT approaches are shown in table 1. The etiology of the cervical lesion was atheromatous disease in 34 patients (85%) and arterial dissection in 6 (15%). Upon admission, 37 patients (93%) were receiving at least one antiplatelet drug, and 7 (18%) received anticoagulant treatment for previous conditions, as well as aspirin.
| Table 1: Characteristics and outcomes of the general population, and group of patients according to the therapeutic approach. | ||||
| Endovascular Treatment | ||||
| Overall (40) | Head-First (22) | Neck-First (18) | p - value | |
| Mean Age ± SD, years | 65 (±13.81) | 68 ± 13.6 | 61.1 ± 13.4 | 0.15 |
| Female | 13 (32.5%) | 9 (40.9%) | 4 (22.2%) | 0.2 |
| Medical history: | ||||
| Hypertension | 23 (58%) | 11 (50%) | 12 (67%) | 0.28 |
| Diabetes | 5 (13%) | 2 (9%) | 3 (17%) | 0.47 |
| Atrial fibrillation | 7 (17.5%) | 4 (18.2) | 1 (6) | 0.39 |
| Hypercholesterolemia | 13 (33.0%) | 8 (36%) | 5 (28%) | 0.56 |
| Current smoking | 13 (33.0%) | 7 (32%) | 6 (33%) | 0.92 |
| Coronariopathy | 10 (25.0%) | 7 (32%) | 3 (17%) | 0.27 |
| Stroke/TIA | 8 (20.0%) | 6 (27%) | 2 (11%) | 0.2 |
| Median admission NIHSS | 13.5 (9.0- 18.0) | 13 (9-18) | 16 (11-19) | 0.71 |
| DWIvol (IQR) | 21.0 (6.1- 63.0) | 14.5 (5-58) | 23 (14.7-64) | 0.21 |
| Onset to groin (IQR) | 298 (220.0-582.0) | 241 (188-492) | 403.5 (224-585) | 0.4 |
| Groin to recanalization (IQR) | 32 (19-54) | 33 (16-54) | 29 (19-65) | 0.97 |
| Median ASPECTS-DWI (IQR) | 6.0 (5.0-8.0) | 7 (5-9) | 5.5 (5.7) | 0.21 |
| mTICI 2b-3 | 33 (82.5%) | 17 (77.3%) | 16 (88.9%) | 0.33 |
| mRS 90 ≤ 2 | 20 (50.0%) | 10 (45.5) | 10 (55.6) | 0.52 |
| mRS 90 6 | 5 (12.5%) | 5 (22.7) | 0 | 0.05* |
| sICH | 4 (10%) | 3 (13.6) | 1 (5.6) | 0.39 |
| TIA: Transient Ischemic Attack. NIHSS: National Institute Health Stroke Scale. DWIvol: Volume of ischemic core. IQR: Interquartile Range. ASPECTS: Alberta Stroke Program Early CT Scores. Median ASPECTS-DW: ASPECTS calculated in DWI. mTICI: modified thrombolysis in cerebral infarction. mRS: modified Rankin Scale. sICH: symptomatic Intracranial Hemorrhage. * p - calculated with Fisher Test | ||||
Endovascular technique and outcomes
A distal approach was used in 22 cases (55%), and proximal in 18 (45%). No significant differences were identified regarding age, medical history, baseline NIHSS, DWIvol, and groin-onset time concerning the therapeutic approach used (Table 1). Emergent cervical carotid artery stenting (CAS) was performed in 97.5% of patients. The 39 patients who were treated with CAS received dual antiplatelet therapy with intravenous ASA loading infusion at the procedure followed by loading of the second antiplatelet immediately thereafter.
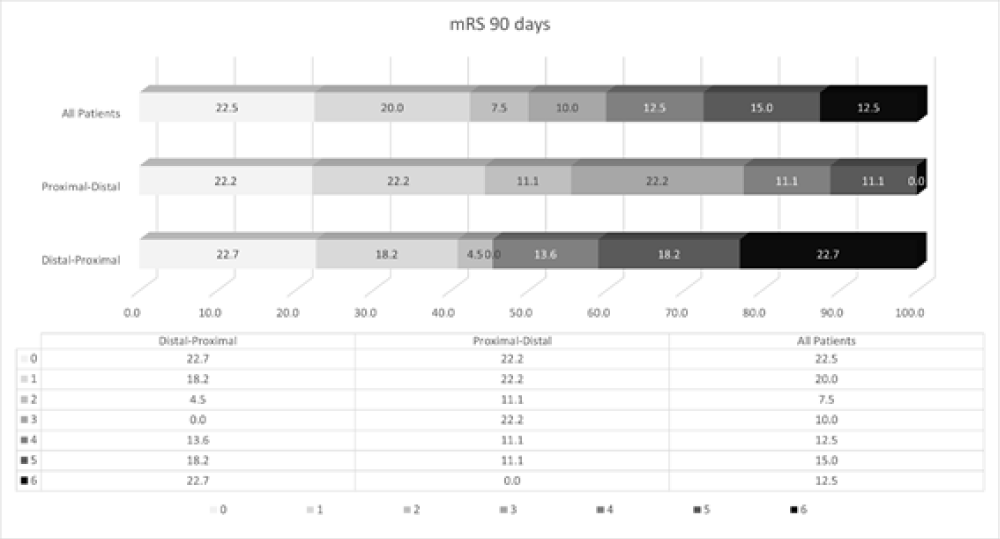
Rates of functional independence at 90 days were similar between groups (p = 0.53), as well as rates of successful reperfusion (mTICI 2b/3) (p = 0.42). These results are portrayed in figure 2. The occurrence of sICH was similar between groups (p = 0.61). At 90-day follow-up, 5 (12.5%) patients died, most being related to clinical complications, delayed referral, or malignant infarcts (Table 2). All these patients received distal thrombectomy first (p = 0.053).
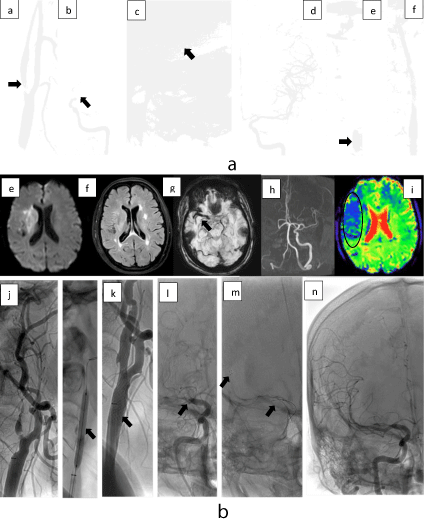
Figure 1: 1a Distal approach. 62 years old patient with acute left T.O. (a) Diagnostic DSA with severe stenosis in the bulbar ICA with floating thrombus, (b) intracranial DSA showed thrombus, (c) Trevo 6/20 mm in the left MCA and ICA, (d) After the First pass, TICI 3 scale was achieved, (e) Balloon angioplasty was done in the ICA, (f) stent angioplasty was done. 1b. Proximal approach. 48 years old patient with acute right T.O. Diagnostic MRI (e) right the DWI showed a basal ganglia and subinsular white matter substance restriction area with (f) mismatch DWI/FLAIR, (g) SWI with thrombus in right M1, (h) The TOF MRA showed lack of signal in the right ICA and MCA s, (i) PWI with Tmax prolongation. (j) Pre dilatation balloon angioplasty was done with 3/15 mm and (k) a 7 mm x 40cm carotid wall was used, (l) in the intracranial DSA proximal M1 was occluded, (m) a solitaire 4/20 mm was used, after two passes, (n) TICI 3 was achieved.
Figure 2: 90-day mRS discriminated by the treatment approach.
| Table 2: Patient characteristics and cause of death. | ||||||||||
| Age | NIHSS | DWIvol | Onset-to-Thrombus | mTICI | PH | sICH | Pre-hospital Treatment | Hospital Treatment | Causes death | |
| #1 | 94 | 18 | 6 | 351 m | 2b | no | no | AAS | Abciximab AAS/Clop | alveolar hemorrhage |
| #2 | 72 | 9 | 5 | 169 m | 2a | PH2 | yes | AAS | tPA AAS/Clop | sICH |
| #3 | 44 | 14 | 169 | 792 m | 3 | PH1 | no | AAS | No | Malignant stroke |
| #4 | 64 | 19 | 112 | 1130 m | 2b | PH1 | yes | AAS | Tirofiban, AAS/Clop | Malignant stroke |
| #5 | 78 | 32 | 7 | 1052 m | 3 | no | no | AAS/warfarin | AAS/Pras | Malignant stroke |
| NIHSS National Institute Health Stroke Scale. DWIvol: Volume of the ischemic core, median. mTICI: modified thrombolysis in cerebral infarction. sICH: symptomatic Intracranial Hemorrhage. PH: Parenchymal Hemorrhage. Clop: Clopidogrel, Pras: Prasugrel. m: minutes | ||||||||||
Use of tPA
Intravenous tPA was used in 22 (55%) patients, without significant differences regarding age, baseline NIHSS, or DWIvol compared to patients who did not receive fibrinolytic treatment. The temporal metrics were longer in the group that did not receive fibrinolytic, with an onset-recanalization time of 559 min (IQR 318-792) vs. 268 min (IQR 180-465) (p = 0.0008). Furthermore, we did not find significant associations between tPA administration and rates of mTICI 2b/3 (p = 0.34), mRS 0-2 (p = 0.53), sICH (p = 0.30), or mRS6 (p = 0.15) (Table 3).
| Table 3: | |||
| Variable | No-tPA Patients n 18 | tPA Patients n 22 | p |
| Age mean (SD) | 66.67 (15) | 63.81 (12.93) | 0.52 |
| NIHSS basal | 17 (9-22) | 12.5 (11-17) | 0.19 |
| DWIvol (IQR) | 22 (6.1-82) | 19 (5.4-58) | 0.51 |
| ASPECTS-DW | 5 (4-8) | 6 (5-8) | 0.74 |
| Onset-to-door median (IQR) | 400 (230-725) | 142.5 (62-374) | 0.001* |
| Onset-to-groin median (IQR) | 527 (283-774) | 224 (168-445) | 0.001* |
| Onset-to-recanalization | 559 (318-792) | 268 (180-465) | 0.0008* |
| Door-to-groin | 65 (42-93) | 95 (61-123) | 0.1853 |
| mRS ≤ 2 | 8 (44.4%) | 12 (54.5%) | 0.53 OR 1.5 (0.42-5.24) |
| mRS 6 | 4 (22.2%) | 1 (4.5%) | 0.15** OR 6.0 (0.60-59.44) |
| sICH | 3 (16.7%) | 1 (4.5%) | 0.30** OR 4.2 (0.39-44.40) |
| mTICI 2b-3 | 16 (88.9%) | 17 (77.3%) | 0.34 OR 2.35 (0.39-13.90) |
| NIHSS basal: National Institute Health Stroke Scale on admission; DWIvol: Volume of ischemic core; ASPECTS-DW: Alberta Stroke Program Early CT Scores calculated in DWI. mTICI: modified thrombolysis in cerebral infarction. sICH: symptomatic Intracranial Hemorrhage. * p: less than the significance level (0.05). mRS: modified Rankin Scale ≤ 2: functional independence, 6: death; ** p calculated with Fisher Test. | |||
Malignant Infarction (MI)
Five patients (12.5%) evolved with MI of the MCA within 48 hours. The score on the mTICI scale after MT was 2b-3 in the 5 patients. Decompressive craniectomy (DC) was performed in 3 of these patients and the mRS at 90 days was 6 in 1 patient and 5 in 2. The mRS at 90 days of the other 2 patients who did not undergo DC was 6.
Despite the challenging technical scenario that represents the endovascular management of TO, our report involving a real-world registry showed that EVT of these complex subset of patients was feasible, with no clear associations between outcomes and the selected approach regarding procedural techniques or adjuvant medical strategies.
Approximately 20% of acute anterior circulation strokes are caused by carotid occlusions or high-grade stenotic lesions of the ipsilateral cervical internal carotid [2,21]. TO strokes represent around 10% - 15% of all AIS undergoing thrombectomy [22,23]. The underlying pathophysiology involves either atherosclerotic disease or a dissection of the proximal vasculature leading to complete occlusion and an embolus causing a distal tandem occlusion [5,24]. Unlike isolated MCA occlusion, the extracranial stenosis/thrombus restricts access to the distal lesion. Therefore, TO are considered challenging lesions to EVT.
Concerning clinical outcomes, we identified a good functional result in half of the patients with TO strokes, with rates of sICH and mortality of 10% and 12.5%, respectively; which were comparable to previous studies [21,25–27].
The challenge with the TO scenario is the presence of blocked access to the distal lesion by the proximal occlusion. There is no doubt about the need for distal recanalization of the intracranial occlusion in TO, although there is a lack of consensus regarding the best management of the acute extracranial lesion. The principle of a distal approach is to treat the symptomatic lesion first and restore perfusion to the involved territory [9]. Once the distal occlusion has been treated, the neurovascular surgeon has time to define the treatment strategy of the proximal lesion to prevent future embolic events. This approach has the advantage of shortening the recanalization time of the intracranial vessel, thus decreasing the duration of ischemia. However, this technique exposes the patient to a possibility of new distal emboli complications during angioplasty and stenting of the extracranial ICA, with the inherent need to repeat the thrombectomy [5]. The opposite approach (proximal) improves these collaterals while distal recanalization is performed [3].
In our report, almost half of the patients received the proximal approach and 55% received the distal approach, at the discretion of the operating interventional neuroradiologist. We did not find any significant associations between these strategies and stroke metrics, and rates of post-procedural TICI 2b-3, sICH, or functional independence. It is noteworthy that all demised patients (n = 5) belonged to the distal group. We believe that these were not related to the procedural strategy since they occurred in 1 patient with alveolar hemorrhage, 3 patients had an LKW-groin time greater than 6 hours, and in 2 patients presenting an ischemic core > 100 mL at admission evolving to malignant infarcts.
Currently, there is no consensus on how to treat proximal carotid lesions in TO. Since emergent carotid stenting requires an appropriate antiplatelet regimen, there have been some concerns regarding the risk of increased periprocedural hemorrhage, leading some authors to consider angioplasty-only at the time of the initial procedure. The use of aspirin in general AIS treated with thrombolysis has been related to higher rates of sICH [28]. We did not find an association between tPA and successful reperfusion, mRS ≤ 2, or the presence of sICH. Such reperfusion failure with fibrinolytics might be related to the fact that most TO are secondary to atherothrombotic disease of the carotid artery with large clot burden and complete hemodynamic compromise. In this regard, the TITAN registry did not find an association between tPA and successful reperfusion [29].
It is noteworthy that, as part of a real-world scenario, our study included patients who received treatment of TO within an extended window. Indeed, 43% of the patients were over the 6-window, without substantial prejudice to their functional or safety outcomes (mRS ≤ 2 = 47%, mRS 6 = 18%).
Although in our experience we did not find any clear benefit associated with the different TO approach used, both techniques might be appropriate according to the particular situation and patient’s anatomy. In the proximal approach, the benefit could be related to early treatment of extracranial ICA, leading to the advantage of improved collateralization attributed to angioplasty of extracranial ICA. Also, the better visualization of the intracranial ICA after proximal angioplasty could favor the advancement of the devices towards the intracranial occlusion in a more secure way [4]. On the other hand, in the case of distal microemboli related to the procedure, these might be extracted during subsequent thrombectomy. In the case of the distal approach, the advantage is the rapid reperfusion obtained by recanalizing the intracranial occlusion first.
Limitations
Several limitations should be acknowledged. This study was carried out in a single-academic center study specialized in the treatment of stroke, therefore extrapolation of our findings to general practice institutions should be cautious. Another limitation is the retrospective, non-controlled design with relatively small sample size. Prospective, randomized clinical studies are warranted to explore whether the proximal approach is related to better clinical outcomes.
In this study, the EVT of stroke-related TO lesions was feasible, with no clear associations between outcomes and the selected approach regarding procedural techniques or adjuvant medical strategies.
- Gregory J, Poppe Alexandre Y, Marilyn L, Nicole D, Gioia Laura C, et al. Lack of Consensus Among Stroke Experts on the Optimal Management of Patients With Acute Tandem Occlusion. Stroke. 2019; 50: 1254–1256. PubMed: https://pubmed.ncbi.nlm.nih.gov/30890115/
- El-Mitwalli A, Saad M, Christou I, Malkoff M, Alexandrov AV. Clinical and sonographic patterns of tandem internal carotid artery/middle cerebral artery occlusion in tissue plasminogen activator-treated patients. Stroke. 2002; 33: 99–102. PubMed: https://pubmed.ncbi.nlm.nih.gov/11779896/
- Rangel-Castilla, L, Rajah GB, Shakir HJ, Shallwani H, Gandhi S, Davies JM, et al. Management of acute ischemic stroke due to tandem occlusion: Should endovascular recanalization of the extracranial or intracranial occlusive lesion be done first? Neurosurg Focus. 2017; 42: E16. PubMed: https://pubmed.ncbi.nlm.nih.gov/28366065/
- Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerve Sur. 2015 7: 170–175. PubMed: https://pubmed.ncbi.nlm.nih.gov/25387730/
- Spiotta AM, Lena J, Vargas J, Hawk H, Turner RD, et al. Proximal to distal approach in the treatment of tandem occlusions causing an acute stroke. J Neurointerve Surg. 2015; 7: 164–169. PubMed: https://pubmed.ncbi.nlm.nih.gov/24561885/
- Cohen JE, Gomori JM, Rajz G, Itshayek E, Eichel R, et al. Extracranial carotid artery stenting followed by intracranial stent-based thrombectomy for acute tandem occlusive disease. J NeuroInterve Surg. 2015; 7: 412–417. PubMed: https://pubmed.ncbi.nlm.nih.gov/24727131/
- Maurer CJ, Joachimski F, Berlis A. Two in One: Endovascular Treatment of Acute Tandem Occlusions in the Anterior Circulation. Clin Neuroradiol. 2015; 25: 397–402. PubMed: https://pubmed.ncbi.nlm.nih.gov/24988990/
- Puri AS, Kühn AL, Kwon HJ, Khan M, Hou SY, et al. Endovascular treatment of tandem vascular occlusions in acute ischemic stroke. J NeuroInterve Surg. 2015 7: 158–163. PubMed: https://pubmed.ncbi.nlm.nih.gov/24578485/
- Mpotsaris A, Bussmeyer M, Buchner H, Weber W. Clinical outcome of neurointerventional emergency treatment of extra- or intracranial tandem occlusions in acute major stroke: Antegrade approach with wallstent and solitaire stent retriever. Clin Neuroradiol. 2013; 23: 207–215. PubMed: https://pubmed.ncbi.nlm.nih.gov/23354342/
- Stampfl S, Ringleb PA, Möhlenbruch M, Hametner C, Herweh C, et al. Emergency Cervical Internal Carotid Artery Stenting in Combination with Intracranial Thrombectomy in Acute Stroke. Am J Neuroradiol. 2014; 35: 741–746. PubMed: https://pubmed.ncbi.nlm.nih.gov/24157733/
- Marnat G, Mourand I, Eker O, Machi P, Arquizan C, et al. Endovascular Management of Tandem Occlusion Stroke Related to Internal Carotid Artery Dissection Using a Distal to Proximal Approach: Insight from the RECOST Study. AJNR. Am J Neuroradiol. 2016; 37: 1281–1288. PubMed: https://pubmed.ncbi.nlm.nih.gov/26965467/
- Steglich-Arnholm H, Holtmannspötter M, Kondziella D, Wagner A, Stavngaard T, et al. Thrombectomy assisted by carotid stenting in acute ischemic stroke management: Benefits and harms. J Neurol. 2015; 262: 2668–2675. PubMed: https://pubmed.ncbi.nlm.nih.gov/26345413/
- Lockau H, Liebig T, Henning T, Neuschmelting V, Stetefeld H, et al. Mechanical thrombectomy in tandem occlusion: Procedural considerations and clinical results. Neuroradiology. 2015; 57: 589–598. PubMed: https://pubmed.ncbi.nlm.nih.gov/25404414/
- Lescher S, Czeppan K, Porto L, Singer OC, Berkefeld J. Acute stroke and obstruction of the extracranial carotid artery combined with intracranial tandem occlusion: Results of interventional revascularization. Cardiovascular Interventional Radiol. 2015; 38: 304–313. PubMed: https://pubmed.ncbi.nlm.nih.gov/25547082/
- Ferguson Gary G, Michael E, Barr Hugh WK, Patrick CG, Barnes Robert W, et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999; 30: 1751–1758. PubMed: https://pubmed.ncbi.nlm.nih.gov/10471419/
- Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet (London, England). 2000; 355: 1670–1674. PubMed: https://pubmed.ncbi.nlm.nih.gov/10905241/
- Barber PA, Hill MD, Eliasziw M, Demchuk AM, Pexman JHW, et al. Imaging of the brain in acute ischaemic stroke: Comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol. 2005; 76: 1528–1533. PubMed: https://pubmed.ncbi.nlm.nih.gov/16227545/
- Purushotham A, Campbell BCV, Straka M, Mlynash M, Olivot JM, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke: Official J Int Stroke Society. 2015; 10: 348–353. PubMed: https://pubmed.ncbi.nlm.nih.gov/23802548/
- Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke. 2013; 44: 2650–2663. PubMed: https://pubmed.ncbi.nlm.nih.gov/23920012/
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New Engl J Med. 2008; 359: 1317–1329. PubMed: https://pubmed.ncbi.nlm.nih.gov/18815396/
- Jadhav AP, Zaidat OO, Liebeskind DS, Yavagal DR, Haussen DC, et al. Emergent Management of Tandem Lesions in Acute Ischemic Stroke. Stroke. 2019; 50: 428–433. PubMed: https://pubmed.ncbi.nlm.nih.gov/30580729/
- Grigoryan M, Haussen DC, Hassan AE, Lima A, Grossberg J, et al. Endovascular Treatment of Acute Ischemic Stroke Due to Tandem Occlusions: Large Multicenter Series and Systematic Review. Cerebrovascular Diseases (Basel, Switzerland). 2016; 41: 306–312. PubMed: https://pubmed.ncbi.nlm.nih.gov/26881779/
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. New Engl J Med. 2015; 372: 2296–2306. PubMed: https://pubmed.ncbi.nlm.nih.gov/25882510/
- Gory B, Piotin M, Haussen DC, Steglich-Arnholm H, Holtmannspötter M, et al. Thrombectomy in Acute Stroke With Tandem Occlusions From Dissection Versus Atherosclerotic Cause. Stroke. 2017; 48: 3145–3148. PubMed: https://pubmed.ncbi.nlm.nih.gov/28974628/
- Goyal M, Menon BK, Zwam WH. van Dippel DWJ, Mitchell PJ, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. The Lancet. 2016; 387: 1723–1731. PubMed: https://pubmed.ncbi.nlm.nih.gov/26898852/
- Gory B, Haussen DC, Piotin M, Steglich-Arnholm H, Holtmannspötter M, et al. Impact of intravenous thrombolysis and emergent carotid stenting on reperfusion and clinical outcomes in patients with acute stroke with tandem lesion treated with thrombectomy: A collaborative pooled analysis. Eur J Neurol. 2018; 25: 1115–1120. PubMed: https://pubmed.ncbi.nlm.nih.gov/29575634/ B
- Sivan-Hoffmann R, Gory B, Armoiry X, Goyal M, Riva R, et al. Stent-Retriever Thrombectomy for Acute Anterior Ischemic Stroke with Tandem Occlusion: A Systematic Review and Meta-Analysis. European Radiology. 2017; 27: 247–254. PubMed: https://pubmed.ncbi.nlm.nih.gov/27085698/
- Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: A randomised controlled trial. The Lancet. 2012: 380: 731–737. PubMed: https://pubmed.ncbi.nlm.nih.gov/22748820/
- Papanagiotou P, Haussen DC, Turjman F, Labreuche J, Piotin M, et al. Carotid Stenting With Antithrombotic Agents and Intracranial Thrombectomy Leads to the Highest Recanalization Rate in Patients With Acute Stroke With Tandem Lesions. JACC. Cardiovascular Interventions. 2018; 11: 1290–1299. PubMed: https://pubmed.ncbi.nlm.nih.gov/29976365/