More Information
Submitted: December 21, 2020 | Approved: December 29, 2020 | Published: December 30, 2020
How to cite this article: Cuspineda-Bravo ER, García- Menéndez M, Castro-Batista F, Barquín-García SM, Cadelo-Casado D, et al. Impact of mandibular advancement device in quantitative electroencephalogram and sleep quality in mild to severe obstructive sleep apnea. J Neurosci Neurol Disord. 2020; 4: 088-098.
DOI: 10.29328/journal.jnnd.1001041
Copyright License: © 2020 Cuspineda-Bravo ER, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Impact of mandibular advancement device in quantitative electroencephalogram and sleep quality in mild to severe obstructive sleep apnea
Cuspineda-Bravo ER1*, García- Menéndez M2, Castro-Batista F1, Barquín-García SM1, Cadelo-Casado D1, Rodríguez AJ3 and Sharkey KM4
1Institute of Neurology and Neurosurgery, La Habana, Cuba
2Hermanos Ameijeiras Hospital, Cuba
3New York University, Department of Neurology, USA
4Alpert Medical School, Brown University, Departments of Medicine and Psychiatry & Human Behavior, USA
*Address for Correspondence: Cuspineda-Bravo ER, Ave 29 #114 esq D. Vedado. Plaza de la Revolución. La, Habana, Cuba, Email: [email protected]; [email protected]
Sleep related breathing disorders (SRBD) are among seven well-established major categories of sleep disorders defined in the third edition of The International Classification of Sleep Disorders (ICSD-3), and Obstructive Sleep Apnea (OSA) is the most common SRBD [1,2]. Several studies have demonstrated that obstructive sleep apnea treatment increases the quality of life in OSA patients [3-8]. Indeed, excessive daytime sleepiness (EDS), cognitive impairment (e.g., deficits in attention-concentration, memory, dexterity, and creativity), traffic accidents, and deterioration of social activities are frequently reported in untreated patients [9-11]. Furthermore, an increase in cardiovascular morbidities and mortality (systemic hypertension, stroke, cardiac arrhythmias, pulmonary arterial hypertension, heart failure) [12], metabolic dysfunction, cerebrovascular ischemic events and chemical/structural central nervous system cellular injuries (gray/white matter) has been reported in OSA patients [13-17].
Continuous positive airway pressure (CPAP) therapy is considered the gold standard for treatment of moderate-severe OSA, nevertheless there is an increasing body of evidence supporting the usefulness of mandibular advancement devices (MADs) for improving quality of life and respiratory parameters even among patients with a high severity of OSA burden [5,10,18,19]. According to the standard of care of the American Academy of Sleep Medicine (AASM), MADs are indicated for mild to moderate OSA particularly in the context of CPAP intolerance or refusal, surgical contraindication, or the need for a short-term substitute therapy [9,15,20-22]. In Cuba, CPAP machines are not readily available; they are expensive and the majority of OSA patients cannot obtain this mode of therapy. Taking into account this problem, our hypothesis was based in the scientific evidences of MAD effectiveness, considering that low cost MADs could offer a reasonable alternative treatment for patients with OSA where CPAP technology are not handy. In this way our purpose was to assess the efficacy of one of the most simple, low cost, manufactured monoblock MAD models (SAS de Zúrich) in terms of improvements in cerebral function, sleep quality and drowsiness reports in a group of Cuban OSA patients with mild to severe disease. Outcome measures included changes in the brain electrical activity, sleep quality, and respiratory parameters, measured by EEG recording with qEEG analysis and polysomnographic studies correspondingly, which were recorded before and during treatment with an MAD, as well as subjective/objective improvements in daytime alertness.
Subjects
Twenty adult patients with OSA diagnosis by full night polysomnographic study (AHI > 5) were selected for MAD treatment (SAS de Zúrich) after dentist specialist evaluation. Dental status that allowed mandibular advancement device treatment included: (1) existence of at least1 molar and 6anterior teeth per jaw; superior and inferior; and (2) the absence of contraindications, such as abnormal dentition, acute dental lesion or periodontal infections, temporomandibular dysfunction, dentures, or front/back teeth mobility issues. All patients underwent a basic orthodontic documentation, a panoramic x-ray, and cephalometric analysis.
Patients were recruited from the Sleep Disorder consultation of the Neurology and Neurosurgery Institute (NNI) in Havana; 16 were males and 4 females; mean age 42.9 years (range 27 to 53 years). Two patients had mild OSA, defined as apnea-hypopnea index (AHI) = 6-15 events/hr, seven were considered moderate (AHI = 16-30 events/hour) and eleven had severe disease (AHI ≥ 31) scored according to AASM critera [23,24]. They were asked to perform a second PSG study 6 month after treatment with the MAD in situ. Basal awake EEG and Epworth sleepiness scale (ESS, for EDS assessment) were recorded before both PSG studies (diagnostic PSG and PSG with MAD treatment) with the same protocol. Exclusion criteria (not related to dental examination) included a history of cerebral damage of any etiology, a history of other sleep disorders or pulmonary diseases, the presence of a neurological or psychiatric condition, obstructive lesion in the upper airway (e.g., tonsillar hypertrophy, enlarged uvula), and/or the use of any medication known to affect sleep, EEG frequency composition, or respiratory function in the month prior to the studies. Data on side effects and compliance were available for all participants. For analyses of PSG, EEG, and daytime functioning, we excluded two patients who had significant weight loss (≥ 10% of body weight loss at the time of the second evaluation) [25,26] and one patient who used MAD therapy for less than 3 weeks.
Informed consent was obtained from all patients.
Procedures
Awake EEG and night-time PSG recordings: EEG was recorded using a 19-channel MEDICID 05 system (Neuronic S.A.), while patients were awake with closed eyes. EEG were recorded using the following technical parameters: gain of 20,000; band-pass filtering between 0.3-30 Hz; 60 Hz “notch” filter; level of noise of 2 μv RMS; sampling period of 5msec.
Nineteen monopolar derivations of the International10/20 System were used for recordings (FP1, FP2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, Pz), referenced to linked left and right mastoid electrodes and grounded at Fpz. Recordings began with 5 min of basal (closed eyes) EEG, followed by 5 alternating 10-sec periods of open eyes (OE) and closed eyes (CE). Movement artifacts were monitored by use of the electro-oculogram (EOG).
Full-overnight PSGs (8-9 hours) were recorded in the NNI sleep laboratory using the same equipment (MEDICID 05) and Neuronic Sleep 7.1 software. For sleep stages scoring frontal, central and occipital EEG electrodes were placed following International 10/20 system as recommended by the American Academy of Sleep Medicine [27] EOG (left and right), submental EMG, vibration sensor for snoring assessment, and electrocardiogram were recorded by superficial electrodes. Thoraco-abdominal bands and thermistors were used to monitor respiratory effort and oro-nasal airflow correspondingly. Oxygen saturation (SaO2) during sleep was measured continuously by a transcutaneous finger pulse oximeter. Left and right leg movements were assessed by piezoelectric sensors.
Overnight staff was instructed to adjust respiratory sensors that were not functioning correctly when the patient woke spontaneously, but otherwise did not intervene during nighttime recordings.
qEEG analysis: For each subject´s EEG, twenty-four artifacts free segments of 2.56 sec. duration were selected by means of visual editing by an expert electroencephalographer (EC), avoiding segments with obvious state changes such as drowsiness. Quantitative EEG analysis using this method yields a stable replicable set of qEEG measures [28]. The Fast Fourier Transform (FFT) and cross-spectral matrices were calculated every 0.39 Hz of the EEG spectrum from 0.39 to 19.11 Hz. After that, broad-band spectral parameters (BBSP): Absolute Power (AP), Relative Power (RP) and Mean Frequencies (MF) were obtained and were analyzed for the 4 classic EEG frequencies bands (delta, theta, alpha and beta) in closed eyes sub-state records. The Absolute Power (AP) characterize the energy content in each frequency band and can be represented graphically as the area under the curve (spectrum)of that frequency band (delta, theta, alpha and beta); it is computed in units of µV2/Hz. Total absolute energy is the sum of the 4 bands absolute energies. The Relative Power (RP) represents the contribution of each band to the total spectrum energy; it is computed by dividing the absolute energy of one specific band by the total absolute energy of the spectrum and is expressed as a percentage. Mean frequencies (MF) are defined as the frequency values that constitute the “gravity center” for one band or for the total spectrum. These centers are the frequencies where the area under the spectral curve is divided in half; it is expressed in Hz.
We also computed BBSP Indices, i.e., AP, RP and MF of slow (delta + theta) to fast (alpha + beta) frequencies indices, that is: delta + theta/alpha + betaindex). Increased slow EEG frequencies and slow-to-fast frequency index is considered a sign of neuronal and network dysfunction when present in wakefulness in adults. The slow-to-fast EEG frequency index has showed to be a useful measure to detect cerebral disturbances in patients with OSAS, cerebrovascular disease and dementia [29-33].
PSG analysis: Scoring of sleep stages was performed manually at 30-sec intervals (sleep epoch), according to the criteria of AASM Manual for the Scoring of Sleep and Associated Events 2007 [27].
Apnea, hypopnea and arousal events were defined according to the 2012 AASM Scoring Rules. Apnea/hypopnea index was defined as the total number of apneas and hypopneas per hour of sleep [23,25,34,35].
Severity of OSA was classified as mild when AHI was between 6-15 events/hour, moderate when 16-30 events/hour, and severe when ≥ 31events/hours occurred [24].
In this study, our main outcome measures for assessing MAD treatment efficacy in sleep quality improvement were: AHI, arousal index, oxygen desaturation index (O2dSa index), OSA severity classification after 6-8 month with MAD in situ (treatment response) and ESS score.
Optimum response to MAD treatment was defined as a resolution of symptoms (daytime sleepiness assessed by Epworth Sleepiness Scale, elimination or significant reduction in objective and subjective snoring frequency and intensity and a reduction in apnea/hypopnea index to ≤ 5/hour; Good response was defined when AHI remained > 5 but ≤ 10/hr with ≥ 50% reduction in AHI and symptom resolution; and partial response when AHI remained > 10/hr but when ≥ 50% reduction in AHI was observed. Treatment failure was defined when AHI was reduced by less than 50%.
Excessive daytime sleepiness (EDS) and Compliance: The presence of EDS was assessed by Epworth Sleepiness Scale and was considered present when the ESS score was > = 10 [36]. Treatment compliance was monitored by diary completion during the 6-8 months of treatment. Patients were asked to check mark only the nights in which MAD was used for more than 4 hours.
Statistical analysis
Statistical analysis of qEEG parameters was performed in two different and complementary ways. For more general data analysis and results, data from all EEG leads were summarized in each patient. That is, for the analysis of BBSP, the average of AP, RP and MF were computed for each frequency band taking into account all EEG leads (i.e: AP average in all the head for delta band before and after MAD). Moreover, a more detailed analysis was also performed independently for each EEG lead to identify the major brain topographies involved in changes pre- and post-MAD treatment.
Similarly, we computed BBSP Indices, i.e., AP, RP and MF (delta + theta/alpha + beta) index, for each patient. In this mathematical formulation, the value of AP, RP and MF for each frequency band corresponded with both the average (av) value computed for all EEG leads (ie: APdelta(av) + APtheta(av)/APalpha(av) + APbeta(av)) for summarizing information, as well as with the specific value of the BBSP for each recorded EEG lead, (ie: APdelta(F3) + APtheta(F3)/APalpha(F3) + APbeta(F3)) before and after MAD treatment to distinguish brain topographies with significant differences in these parameters.
Statistical differences in BBSP and BBSP indices of basal EEG recordings before and after MAD treatment, as well as between PSG studied parameters and Epworth Sleepiness Scale scoring, were computed using the Wilcoxon matched pairs test. For all comparisons, a difference was considered significant when p < 0.05. Results were presented in box & whisker plot graphs (mean ± SD). The database and statistical analysis was performed using Statistic 8.0 StatSoft package.
Procedures for MAD manufacture and fitting
SAS de Zurich is a rigid bimaxillary monobloc MAD constructed with two acrylic plates, upper and lower, similar to two Michigan splints. The plates are connected to each other by mean of rectangular wires folded into a W shape. The anchoring of the apparatus is reinforced by Adams hooks arranged between the first premolars and first molars [37].
MADs were custom manufactured. For each individual patient the initial setting aimed to obtain 75% of mandible maximum protrusion with an inter-arches distance of 5-6 mm at the pre- molar level. If patient experienced discomfort at these settings, the mandibular advance was reduced by 50%.
Informed consent and ethical aspects: Detailed information about the characteristics of the study, procedures and treatment were provided to all studied patients, after that, written informed consent was obtained.
This study was analyzed and approved by the NNI ethic committee.
Results are based on the analysis of data from 17 OSA patients; data from 3 patients were excluded (2 for significant changes in body weight and 1 for early treatment abandon, see methodology).
Comparison of qEEG measures before and after MAD
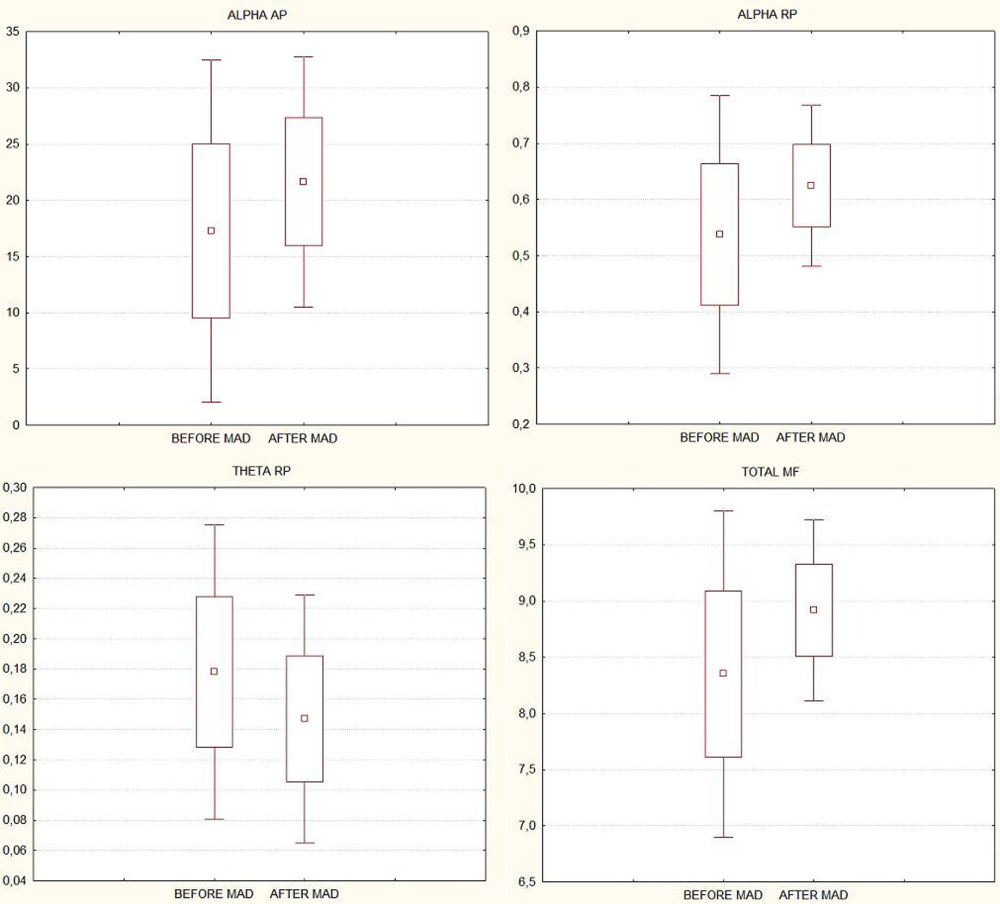
Considering the averaged BBSP (AP, RP and MF) for each frequency band across all EEG leads, statistically significant changes were found in theta and alpha bands. Theta RP diminished (p = 0.03); whereas alpha band AP and RP increased after MAD treatment (p = 0.02, for both measures). Total MF also increased significantly (p = 0.03) (Figure 1).
Figure 1:
When this analysis was performed independently for each EEG lead, we found that the significant increases of alpha AP were topographically located in frontal and occipital left-right leads (p < 0.05), while for alpha RP these changes were observed in all topographical areas (p < 0.05 in all EEG leads).
Significant decreases were found in slow frequency bands; theta RP diminished specifically in frontal, temporal, and occipital topography (both cerebral hemispheres, p < 0.05), whereas significant reduction in delta AP and RP were identified only in the left hemisphere (central, parietal, temporal and occipital regions).
The mean frequencies increases (p < 0.05) were found mainly in left and right temporo-occipital cerebral topography.
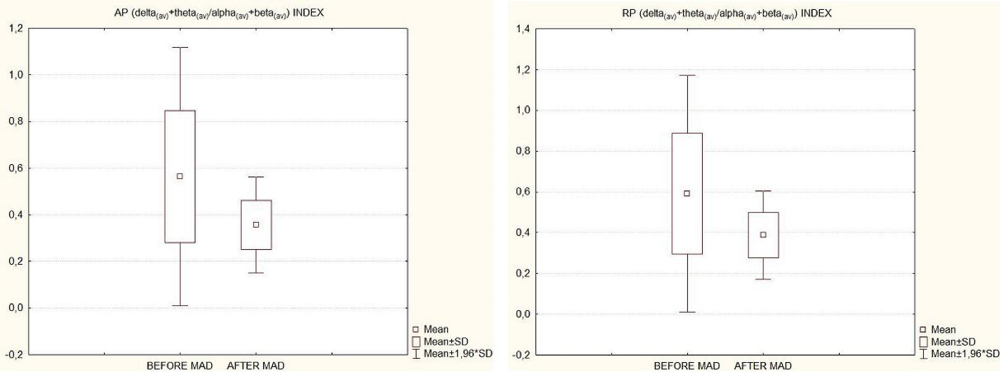
Correspondingly, significant changes in qEEG indices were also found. Both AP and RP delta + theta/alpha + beta indices diminished after 6 month of MAD treatment across all the brain topography (all EEG leads; p < 0.05). Figure 2 shows box & whisker plot graphs (mean ± SD) of averaged (all EEG leads pooled) AP and RP indices (delta(av) + theta(av)/alpha(av) + beta(av)) before and after 6 months of MAD treatment.
Figure 2:
Changes in PSG parameters and epworth sleepiness scale after MAD
The subjective evaluation of snoring (loudness and frequency), daytime sleepiness, and nocturnal apnea events showed that both patients and their bed partners perceived a reduction in these parameters; all of them reported treatment satisfaction, even when symptoms were reduced and not completely eliminated.
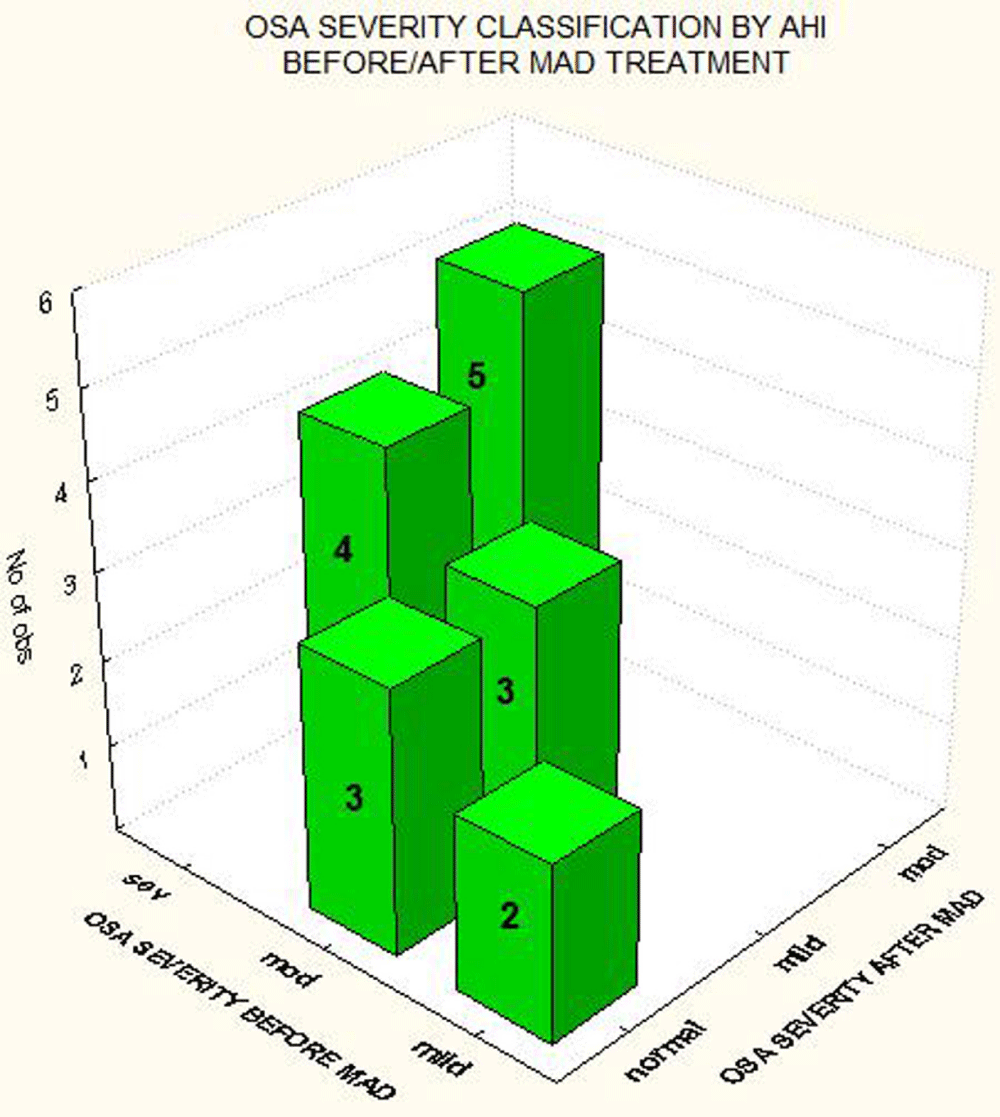
With respect to OSA severity classification, we found that the 2 patients with mild OSA normalized their AHI (≤ 5 events/hours) after 6 month of MAD treatment; 3 of 6 patients with moderate OSA normalized their AHI and the other 3 were classified as having mild OSA in the second PSG study. Regarding patients with severe OSA at baseline, after 6 months of MAD treatment, 4 of 9 patients (44.4%) were classified as mild, and 5 (55.6%) as moderate based on an AHI between 16-30 events per hour with MAD in situ (Figure 3).
Figure 3:
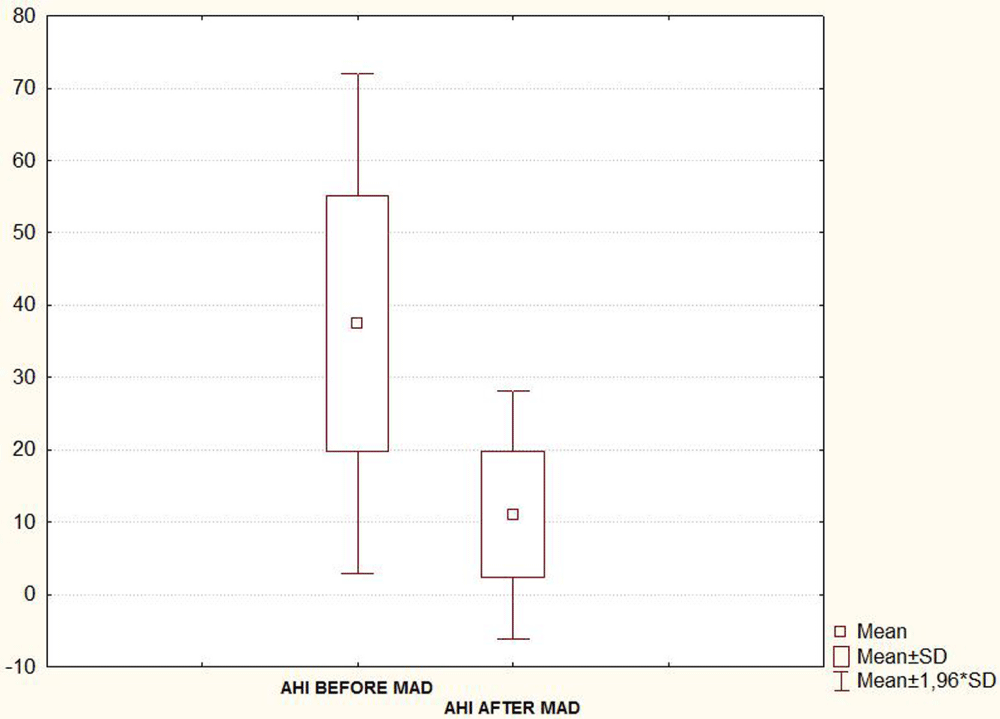
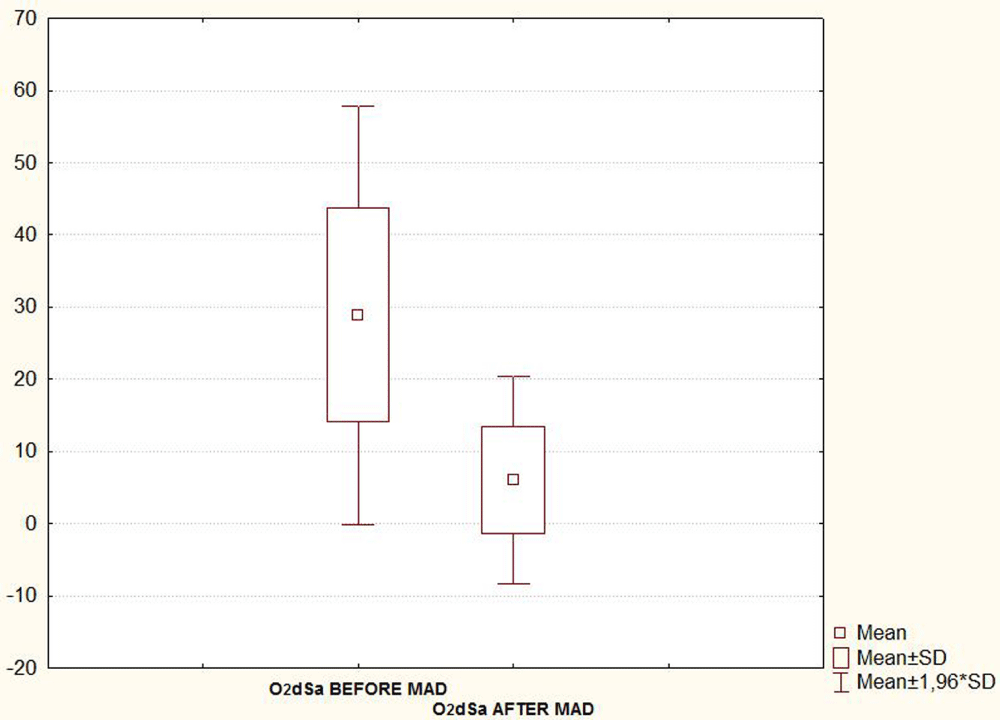
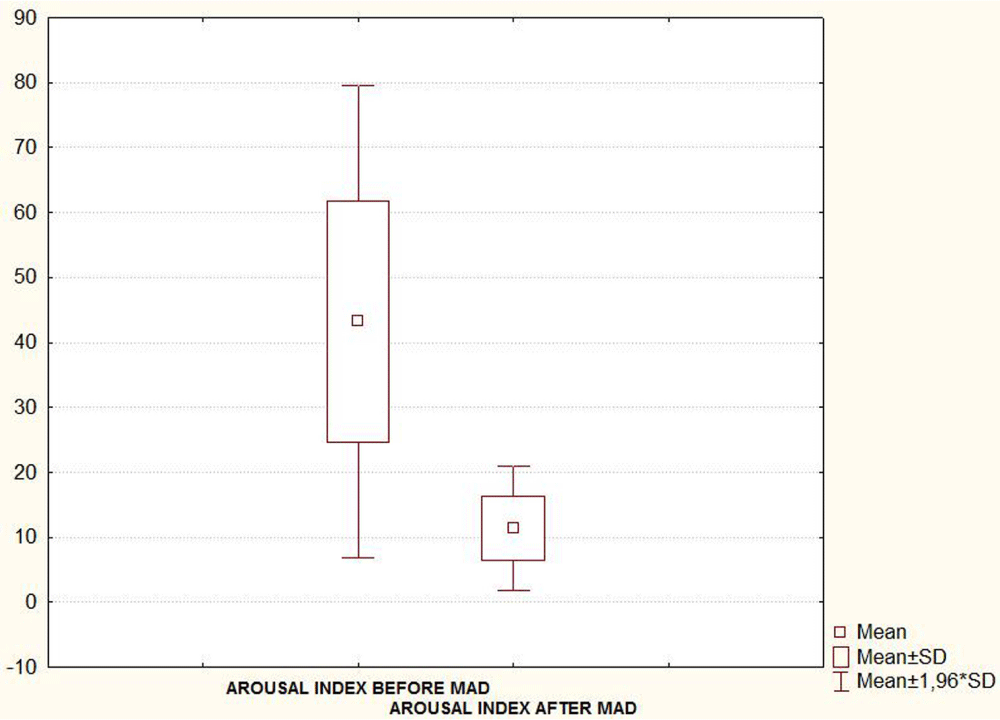
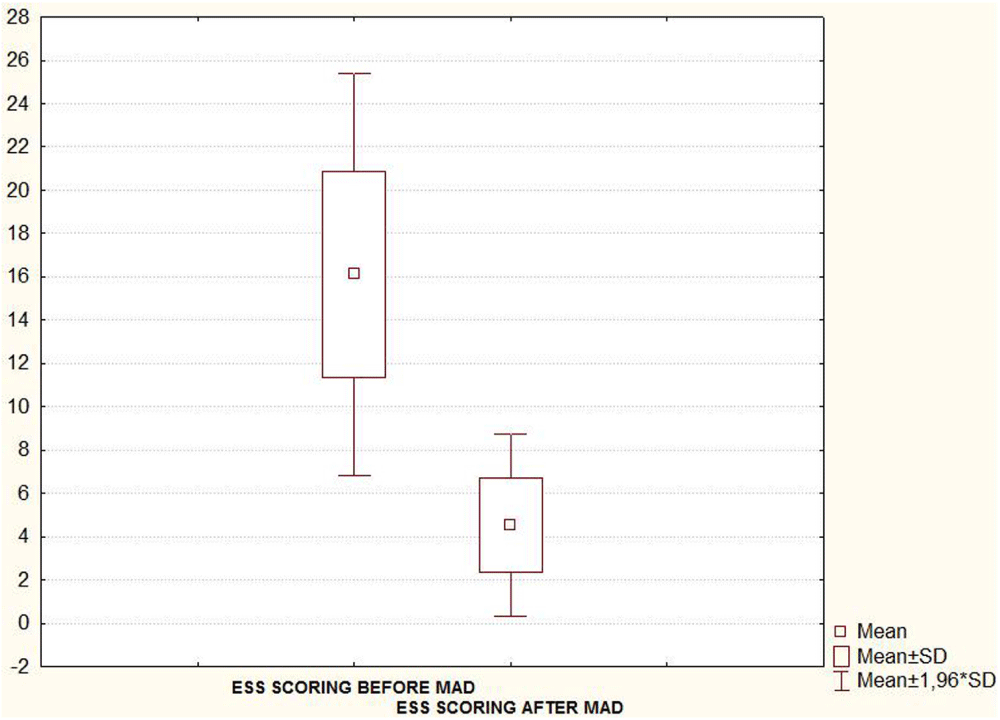
Taking into account the seventeen patients, the mean (SD) AHI, Arousal index and oxygen desaturation (O2dSa) index showed a statistically significant decrease when PSG was recorded with MAD in situ 6-8 month after treatment initiation (Table 1 and Figures 4A-C). A statistically significant decrease in subjective report of excessive daytime sleepiness assessed by the Epworth Sleepiness Scale (ESS) was also found in the second evaluation (Figure 4D, Table 1).
Figure 4A:
Figure 4B:
Figure 4C:
Figure 4D:
| Table1: PSG parameters and ESS score (mean ± SD values) before versus after MAD treatment. Seventeen patients included in the final statistical analysis. | |||
| Parameters | Before MAD mean ± SD |
After MAD mean ± SD |
p - level |
| AHI | 37.4 ± 17.6 | 11.1 ± 8.7 | < 0.05 |
| O2dSa INDEX | 28.9 ± 14.8 | 6.0 ± 7.4 | < 0.05 |
| AROUSAL INDEX | 43.2 ± 18.6 | 11.4 ± 4.9 | < 0.05 |
| ESS SCORE | 16.1 ± 4.7 | 4.5 ± 2.1 | < 0.05 |
| Note: AHI: Apnea/Hipopnea Index; O2dSa: Oxygen desaturation Index; ESS: Epworth Sleepiness Scale. | |||
We also analyzed the data for the moderate and severe patient groups separately and found a significant decrease in AHI, arousal index, and oxygen desaturation index for both groups. Similar analyses were not possible for the mild OSA group because of the small number of patients (2 patients) but important reductions in AHI, arousal index, and oxygen desaturation index were observed in this group as well (Table 2).
| Table 2: Differences in PSG parameters (mean ± SD values) before versus after MAD treatment by OSA severity. Seventeen patients included in the final statistical analysis. | |||||||
| Before MAD | After MAD | ||||||
| OSA SEVERITY | No. Patients | AHI mean ± SD | O2dSa mean ± SD | Arousal mean ± SD |
AHI mean ± SD | O2dSa mean ± SD | Arousal mean ± SD |
| MILD AHI=6-15/hr |
2 | 13.3 ± 2.2 | 8.8 ± 2.7 | 19.7 ± 3 | 2.2 ± 1.1 | 1.7 ± 0.5 | 4.7 ± 1 |
| MODERATE AHI=16-30/hr |
6 | 23.1 ± 4.3 | 19.6 ± 7.1 | 30.8 ± 8.1 | 6.2 ± 2.1 | 2.5 ± 2.6 | 7.7 ± 2.6 |
| SEVERE AHI>=31/hr |
9 | 52.4 ± 7.8 | 39.5 ± 10.6 | 56.7 ± 13.8 | 16.1 ± 9.3 | 9.4 ± 8.7 | 13.6 ± 5 |
| Note: AHI: Apnea/Hipopnea Index, O2dSa: Oxygen desaturation Index | |||||||
For comparison with other similar studies, MAD treatment effectiveness was classified as optimum response; good response, partial response, and treatment failure (see methodology for classification parameters). When examined this way, we found that although sixteen of seventeen patients (94.1%) experienced > 50% reduction of AHI (i.e., presence of treatment response), only 5 patients (29.4%) had an AHI reduction to less than or equal 5 events/hours (patients with optimum response); a good response was found in 6 patients (35.3%), a partial response in 5 (29.4%) and treatment failure (< 50% of AHI reduction) in 1 patient (5.9%).
The patient who was classified as a treatment failure was a man with a BMI of 43 Kg/m2. His AHI was 57.8 events per hour at baseline and 29.3 events/hour with MAD in situ (6 month of treatment). Thus, his AHI decreased by 49.3%).
In summary, if treatment success is analyzed while taking into account OSA severity at the baseline, all of patients with mild and moderate OSA had an optimum or good response; 8 of 9 patients (88.9%) with severe OSA diminished their AHI in more than 50% (3 good responses, 5 partial responses), and 1 patient of this group (11.1%) was considered as treatment failure although an important decrease in AHI was found (Table 3).
| Table 3: MAD treatment effectiveness. Seventeen patients included in the final statistical analysis. | ||||
| Before MAD | Response To MAD | |||
| OSA Severity | Optimal | Good | Partial | Failure |
| Mild | 2 (100%) | - | - | - |
| Moderate | 3 (50%) | 3 (50%) | - | - |
| Severe | - | 3 (33.3%) | 5 (55.6%) | 1 (11.1%) |
| All Patients | 5 (29.4%) | 6 (35.3%) | 5 (29.4%) | 1 (5.9%) |
Weight
No significant differences in body weight values from the date of treatment commencement and date of MAD efficacy assessment occurred in the 17 patients analyzed; therefore variation in patient weight did not influence the results of the study (see exclusion criteria for data analyses).
Side effects
Considering all 20 patients recruited at the beginning of the study (including the 2 with significant changes in body weight and the patient who did not adhere to MAD therapy), the following side effects were reported: dental sensitivity: 5 patients; excessive salivation: 3 patients; muscular and temporo-mandibular joint discomfort: 5 patients. In general, side effects were temporary and most patients overcame them during the 1-3 months of wearing the MAD. Only one patient discontinued MAD therapy and dropped out of the study because of temporo-mandibular joint discomfort.
For compliance analyses we utilized data from all 20 patients. Seventeen of the twenty studied patients (85%) were classified as having good compliance; they met the criteria for “regular MAD user” [9,38-40] completing > 4 hours on ≥ 70% of studied nights using OA treatment (> 4 hours on ≥ 21 days during a 30-day period throughout the analyzed 6-8 months).Two patients (10%) were classified as poor compliance (active treatment for > 4 hours on 15-20 nights during a month), and one patient (5%) gave up the treatment on the first few weeks (non-adherent).
qEEG analysis in OSA patients
This study showed improvements in daytime cerebral function through quantitative analysis of EEG in OSA patients after MAD treatment (increases in fast and decrease in slow frequency bands measures). This improved across a wide range of sleep apnea severity, including among patients who would typically not be considered good candidates for MAD therapy due to the severity of their SRBD. Despite a number of studies showing qEEG parameters changes after CPAP treatment in OSA patients (mainly reporting return to normal values [29,30,41,42], we are not aware of any other studies of qEEG changes after using MAD treatment for OSA.
Our results are comparable with those reported using CPAP therapy. For instance, in a study conducted by Morrison, et al. 2001, the authors found significant decreases in slow activity (delta and theta AP) as well as in slow/fast activity ratio (delta + theta/alpha + beta) for all brain regions pooled (19 EEG electrodes, wakefulness state) after 6 month of CPAP treatment in 14 moderate-severe OSA patients. When these analyses were achieved separately by different cerebral regions (frontal, central, parietal, temporal and occipital), the authors also reported significant decreases in absolute delta and theta activity in all brain areas. Unlike the present study, which showed greater improvements in the left hemisphere for some measures, Morrison and colleagues did not analyze hemispheres separately.
Lee, et al. 2012 [41] also found the presence of changes in wakefulness qEEG parameters after 3 months of CPAP treatment in thirteen patients with severe OSA. They reported significant decreases of EEG slowing across all cerebral regions accompanied by improvement of cognitive functions involving several brain areas. The authors stressed these results suggest that CPAP can induce improvement of cerebral function in OSAS without regional specificity.
Grenèche, et al. 2011 [43] reported a partial improvement of waking EEG abnormalities in moderate-severe OSA patients after 3 month of CPAP therapy; they found a reduction in theta activity while increases in beta activity persisted, suggesting that efforts to stay awake remain strong in these patients.
Nevertheless Xiromeritism, et al, 2011 [13] reported significant slowing increases detected by wakefulness qEEG analysis only for severe OSA patients when they are compared to those observed in healthy subjects (not for patients with mild or moderate OSA), and in contrast with the studies described above, they found significant decreases in theta and alpha relative power with increases in beta and delta relative power after 6-month CPAP treatment. The authors suggested that in severe OSA patients, hypoxia might be responsible for a brain dysfunction (indicated by no improvements in alpha and delta bands) which could not be restored after 6 month of CPAP therapy.
The increments of EEG slowing and reduction in fast frequencies found by qEEG analysis of untreated OSA patients reported in this and a number of studies, have been attributed to sleep fragmentation, daytime sleepiness, and/or hypoxia during sleep [13,41,44-46].
Sleep fragmentation may affect the restorative functions of sleep; the study of Mathieu, et al. 2007 [47] supports the hypothesis that diffuse cortical slowing is a manifestation of sleep fragmentation. They found a significant relationship between EEG slowing ratio in all investigated cortical areas and sleep fragmentation indexed by microarousal; on the other hand, the studies of Sforza, et al. 2002 and Morisson, et al. 1998 [30,48] reported no significant relationship between waking EEG powers and any markers of sleep fragmentation, which the authors interpret as an indication that sleep fragmentation has little, if any role in the occurrence of EEG slowing.
Although there are no well-established relationships between qEEG parameters and objectively-measured sleepiness to date, correlation between subjective daytime sleepiness and increased EEG slowing during wakefulness has been reported. Grenèche, et al. 2008 and Finelli, et al. 2000 [49,50] found a significant correlation between subjective sleepiness and increments of EEG slowing in healthy sleep-deprived subjects. Studies in OSA patients show inconsistent results: while some authors report a significant correlation between sleepiness and awake qEEG parameters(lower relative alpha power and higher relative delta and theta power) with improvements after CPAP treatment (reduction of EEG slowing correlated with decreases of subjective sleepiness) [13,41,51,52], other studies report no significant relationships between waking EEG measures and sleepiness [29,30,43,48].
One interpretation of these discrepant findings is that nocturnal hypoxia, rather than sleep fragmentation and daytime sleepiness, seems to be the underlying mechanism of EEG slowing in OSA patients [43]. Many authors highlight that the presence of functional cerebral impairment demonstrated by qEEG analysis (diffuse increases in slow frequencies) occurs in relation with changes in hemodynamic and cerebral oxygenation during apneic/hypopneic events (reduced arterial oxygen saturation and cerebral tissue hypoxia) that cannot be offset even by increases in cerebral blood flow [30,44,47,54-56]. Moreover significant associations between apnea–hypopnea index and metabolic impairment severity in cerebral white matter have been also reported in OSA patients. It is suggested that these frequent respiratory events can also disrupt the restorative cellular processes leading to cerebral dysfunction and EEG slowing [57].
Moreover, in healthy subjects, studies conducted in hypobaric and hypoxic conditions generated by a low pressure chamber, have shown to induce an increase in relative delta and theta activity and a decrease in relative alpha activity [58,59].
Nevertheless a recent investigation by Wang, et al. 2015 [60] on the effects of hypoxia and/or hypercapnia on electroencephalogram demonstrated that hypercapnia, not hypoxia, seemed to be the critical factor in EEG slowing. However with mixed hypercapnia and hypoxia, a stronger EEG slowing effect was found than when hypercapnia alone, suggesting a potential additive effect of hypoxia on top of hypercapnia in slowing brain activity. This study was conducted in healthy volunteers subjected to controlled hypoxia and/or hypercapnia.
We agree with other authors about the presence of a complex interaction between hypercapnia, hypoxia, sleep fragmentation, sleepiness and cerebral blood flow changes as a constellation of underlying pathophysiological mechanisms, with differential contributions of each factor, on slowing EEG in OSA patients [55,61].
The diffused rather than regional sign of EEG dysfunction found in this work could be related to the presence of extensive structural and functional changes reported in previous brain imaging studies in OSA patients. Altered grey and white matter density with compromised structural/functional integrity across multiple brain regions (frontal and parietal cortex, temporal lobe, anterior cingulate, hippocampus, and cerebellum) have been reported [44,62-65]. Importantly, recent findings have shown functional improvement, increase of gray-matter volume and structural plasticity of brain after OSA treatment with CPAP [66,67]. Our findings extend this work to suggest treatment with MADs may also improve cerebral functioning.
PSG parameters and Epworth Sleepiness Scale scoring, Comparisons before and after treatment with MAD
Comparisons of PSG parameters before and after CPAP treatment in OSA has been shown to significantly reduce AHI and oxygen desaturations with improvement in sleep quality (e.g, decreased arousals, restoration of sleep architecture). Similar results have been described for OSA symptoms such as EDS, for instance, normalization of Epworth Sleepiness Scale scores is often reported [10,40,68].
In the last decades Oral Appliances (OA) including MADs, has emerged as viable alternatives to CPAP machines. Several studies have been carried out for determining its effectiveness and compliance in comparison with CPAP, nowadays OA constitute an effective and well-accepted form of OSA treatment [5,6,9,38].
Phillips, et al. 2013 [5], compared the effectiveness of MAD and CPAP one month after treatment in 108 OSA patients (moderate to severe) in terms of health outcomes. They found that CPAP was significantly more effective than MAD for reducing the apnea-hypopnea index, but compliance was significantly greater with MAD; moreover they found similar results (no statistical differences) between the 2 treatments with respect to improvements in blood pressure, daytime sleepiness, driving stimulator performance, and disease-specific quality of life; Furthermore, MAD was superior to CPAP on several quality-of-life domains and the overall mental component score.
Moreover the study by Doff, et al. [10], provided valuable evidence regarding the long-term relative efficacy of CPAP and MAD for mild to severe OSA treatment. In their 2-years randomized trial including 103 patients, they found no statistical difference between the proportion of patients obtaining successful treatment with MAD and CPAP (56% vs. 60% in non-severe, and 50% vs. 74.1% in severe for MAD and CPAP, respectively). In this study “successful treatment” was defined when AHI was less than 5 events/hour or showed a substantial reduction (at least 50% from the baseline value to a value of < 20 in a patient without subjective OSA symptoms while using therapy). It is important to emphasize, however, that patients had significantly lower AHI and higher oxyhemoglobin saturation levels with CPAP, while both therapies showed substantial improvements in neurobehavioral outcomes.
In a recent comparative (MAD vs. CPAP) longer-term study (5 years) conducted by Anandam, et al. 2013 [69] the authors reported a similar effectiveness in reducing risks of fatal cardiovascular events in severe OSA patients for both therapies.
Despite inferiority of MAD efficacy in diminishing the AHI, there are various trials showing no differences between the efficacies of both treatments in mitigating cardiovascular disturbances such as blood pressure elevation, with improvements of endothelial function, and microvascular reactivity [70-73].
The goal of our study was not to compare the efficacy of CPAP versus MAD because of the non-availability of CPAP in our country, but rather to assess the utility of MAD in settings where CPAP is unavailable or unacceptable to the patient. We found a high success rates with MAD even using a simple monobloc design (SAS Zúrich). As was presented in results, 64.7% of all patients (mild to severe OSA) had an optimum or good outcomes (AHI ≤ 10), and 94.1% experienced a decrease in their AHI of more than 50%; only one patient (with severe OSA and obesity) was recorded as experiencing treatment failure, representing just 5.9% of all patients. Nevertheless, a significant reduction in this patient’s AHI was observed (49.3%).
Similar results were found by Milano, et al. 2013, they studied the efficacy of a two piece MAD device after 6 months of treatment in forty-two mild- to-severe OSAS patients. They found a statistical significant treatment response related to the apnea/hypopnea index and oxygen desaturation index, reporting an optimum therapy response (AHI < 5) in 53% of patients (40% in severe OSAS) and a good response (AHI < 10) in 73% of patients (50% in severe OSAS), but they emphasized the important role of a multidisciplinary patient selection in order to obtain high rates of therapy response with MAD.
Other MAD treatment studies have reported similar success rates: 50% to 80% of mild-severe OSA patients showed significant improvement in the AHI when compared with baseline (before treatment) data. Despite different criteria for defining “success or effectiveness” of the therapy, in most studies “success” or “good” responses have been considered when normalization of AHI (< 5 events/hours) occurs, or when <10 events/hours with > 50% in AHI reduction is found [20,21,74], other authors report “success” when AHI is less than 20 events/hours and > 50% of AHI reduction has been observed [75].
Appropriate patient selection is clearly important to obtain these success rates.
For instance, our study and others have shown that a high BMI seems to interfere negatively in the performance of OA devices. This important consideration has been stressed by many authors reporting BMI as a consistent predictor of MAD efficacy [6,20,76] have suggested that BMI greater than 35 kg/m2 should be a contraindication for MAD therapy. Whether oral appliance therapy mitigates risk of untreated OSA even among patients in whom optimal outcomes are not achieved is a topic worthy of further study.
Final remarks: Despite recommendations in the most recent clinical practice guidelines for the management of OSA in adults, published by the American Academy of Sleep Medicine (AASM), the American Thoracic Society (ATS), and the American College of Physicians (ACP) [25,77,78] suggesting that all patients diagnosed with OSA should be offered positive airway pressure as initial therapy, recent literature, including the present study, provide evidence that MAD is an effective alternative therapy to CPAP, except for in extremely obese patients.
We agree that CPAP is the standard of care, however, we are in agreement with others researchers that a well-prescribed mandibular advance device, correctly manufactured and periodically monitored, can be a highly efficient treatment independent of OSA severity, and particularly in settings where CPAP is not possible [5,15,76,79].
Moreover, MAD therapy is a simple and noninvasive treatment, and because of the small size, MADs are easily portable as compared with CPAP and do not represent a major expense to patients or to the health care system [20,75,80,81]. These characteristics make MAD an important treatment option not only for patients with non-adherence to CPAP but for patients in regions where CPAP machines are not available because complexity and cost [74-76,82].
The authors thank to the Stomatology Faculty of the Havana Medical University, for collaborate with this scientific project.
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014; 146: 1387-1394. PubMed: https://pubmed.ncbi.nlm.nih.gov/25367475/
- Judd BG, Sateia MJ. Classification of sleep disorders. 2015. www.uptodate.com
- Naismith SL, Winter VR, Hickie IB, Cistulli PA. Effect of oral appliance therapy on neurobehavioral functioning in obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2005; 1: 374-380. PubMed: https://pubmed.ncbi.nlm.nih.gov/17564405/
- Blanco J, Zamarron C, Abeleira Pazos MT, Lamela C, Suarez Quintanilla D. Prospective evaluation of an oral appliance in the treatment of obstructive sleep apnea syndrome. Sleep Breath. 2005; 9: 20-25. PubMed: https://pubmed.ncbi.nlm.nih.gov/15785917/
- Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, et al. Health outcomes of CPAP versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013; 187: 879-887. PubMed: https://pubmed.ncbi.nlm.nih.gov/23413266/
- Gagnadoux F, Fleury B, Vielle B, Pételle B, Meslier N, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009; 34: 914-920. PubMed: https://pubmed.ncbi.nlm.nih.gov/19324954/
- Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Longterm follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011; 82: 162-168. PubMed: https://pubmed.ncbi.nlm.nih.gov/21454959/
- Gauthier L, Laberge L, Beaudry M, Laforte M, Rompre PH, et al. Mandibular advancement appliances remain effective in lowering respiratory disturbance index for 2.5-4.5 years. Sleep Med. 2011; 12: 844-849. PubMed: https://pubmed.ncbi.nlm.nih.gov/21925942/
- Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014; 10: 215-227. PubMed: https://pubmed.ncbi.nlm.nih.gov/24533007/
- Doff MHJ, Hoekema Am Wijkstra PJ, van der Hoven JH, Slater JJRH, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013; 36: 1289-1296. PubMed: https://pubmed.ncbi.nlm.nih.gov/23997361/
- Tripathi A, Gupta A, Tripathi S, Dubey A. A novel use of complete denture prosthesis as mandibular advancement device in the treatment of obstructive sleep apnea in edentulous subjects. JDSM. 2014; 1: 115–119.
- Strohl KP. Overview of obstructive sleep apnea in adults. 2015. www.uptodate.com
- Xiromeritis AG, Hatziefthimiou AA, Hadjigeorgiou GM, Gourgoulianis KI, Anagnostopoulou DN, et al. Quantitative spectral analysis of vigilance EEG in patients with obstructive sleep apnoea syndrome: EEG mapping in OSAS patients. Sleep Breath. 2011; 15: 121–128. PubMed: https://pubmed.ncbi.nlm.nih.gov/20174876/
- Kamba M, Inoue Y, Higami S, Suto Y, Ogawa T, et al. Cerebral metabolic impairment in patients with obstructive sleep apnoea: an independent association of obstructive sleep apnoea with white matter change. J Neurol Neurosurg Psychiatry. 2001; 71: 334–339. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1737534/
- Kryger MH, Malhotra A. Management of obstructive sleep apnea in adults. 2015. www.uptodate.com
- Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009; 6: e1000132. PubMed: https://pubmed.ncbi.nlm.nih.gov/19688045/
- Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008; 31: 1079-1085. PubMed: https://pubmed.ncbi.nlm.nih.gov/18714779/
- Milano F, Mondini S, Billi MC, Gobbi R, Gracco A, et al. The impact of a multidisciplinary approach on response rate of mandibular advancing device therapy in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013; 33: 337-342. PubMed: https://pubmed.ncbi.nlm.nih.gov/24227900/
- Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral Appliance Therapy Improves Symptoms in Obstructive Sleep Apnea. A Randomized, Controlled Trial. Am J Respir Crit Care Med. 2002; 166: 743–748. PubMed: https://pubmed.ncbi.nlm.nih.gov/12204875/
- Dal-Fabbro C, Chaves CM Jr., Bittencourt LRA, Tufik S. Clinical and polysomnographic assessment of the BRD Appliance in the treatment of Obstructive Sleep Apnea Syndrome. Dental Press J. Orthod. 2010; 15: 107-117.
- Liu Y, Lowe AA, Fleetham JA, Park YC. Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2001; 120: 639-647. PubMed: https://pubmed.ncbi.nlm.nih.gov/11742309/
- Bittencourt LRA. Diagnóstico e tratamento da Síndrome da Apnéia Obstrutiva do Sono (SAOS)-Guia Prático. 1ª ed. São Paulo: Médica Paulista. 2008.
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012; 8: 597-619. PubMed: https://pubmed.ncbi.nlm.nih.gov/23066376/
- Kline LR. Clinical presentation and diagnosis of obstructive sleep apnea in adults. 2015. www.uptodate.com
- Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2009; 5: 263-276. PubMed: https://pubmed.ncbi.nlm.nih.gov/19960649/
- Lloberes P, Durán-Cantolla J, Martínez-García MA, Marín JM, Ferrer A, et al. Diagnosis and treatment of sleep apnea-hypopnea sindrome. Arch Bronconeumol. 2011; 47: 143-156. PubMed: https://pubmed.ncbi.nlm.nih.gov/21398016/
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification, 1st ed, American Academy of Sleep Medicine, Westchester. 2007.
- Machado C, Cuspineda E, Valdés P, Virues T, Llopis F, et al. Assessing Acute Middle Cerebral Artery Ischemic Stroke by Quantitative Electric Tomography. Clin EEG Neurosci. 2004; 35: 116-124. PubMed: https://pubmed.ncbi.nlm.nih.gov/15259617/
- Morisson F, Décary A, Petit D, Lavigne G, Malo J, et al. Daytime Sleepiness and EEG Spectral Analysis in Apneic Patients Before and After Treatment With Continuous Positive Airway Pressure. Chest. 2001; 119: 45-52. PubMed: https://pubmed.ncbi.nlm.nih.gov/11157583/
- Morisson F, Lavigne G, Petit D, Nielsen T, Malo J, Montplaisir J. Spectral analysis of wakefulness and REM sleep EEG in patients with sleep apnoea síndrome. Eur Respir J. 1998; 11: 1135–1140. PubMed: https://pubmed.ncbi.nlm.nih.gov/9648968/
- Sheorajpanday RVA, Nagels G, Weeren JTM, De Deyn, PP. Quantitative EEG in ischemic stroke: Correlation with infarct volume and functional status in posterior circulation and lacunar syndromes. Clin Neurophysiol. 2011; 122: 884-890. https://pubmed.ncbi.nlm.nih.gov/20870455/
- Finnigan S, Wong A, Read S. Defining abnormal slow EEG activity in acute ischaemic stroke: Delta/alpha ratio as an optimal QEEG index. Clin Neurophysiol. 2016; 127: 1452-1459. PubMed: https://pubmed.ncbi.nlm.nih.gov/26251106/
- Petit D, Lorrain D, Gauthier S, Montplaisir J. Regional spectral analysis of the REM sleep EEG in mild to moderate Alzheimer’s disease. Neurobiol Aging. 1993; 14: 141–145.
- Millman RP, Kramer NR. Polysomnography in obstructive sleep apnea in adults. 2015. www.uptodate.com
- Strohl KP. Sleep related breathing disorders in adults: Definitions. 2015. www.uptodate.com
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991; 14: 540-545. PubMed: https://pubmed.ncbi.nlm.nih.gov/1798888/
- Macías E, de Carlos F, Cobo J, Díaz B. Aparotología intraoral en el tratamiento de la apnea-hipopnea obstructiva del sueño (SAHOS). RCOE. 2002; 7: 391-402.
- Sutherland K, Phillips CL, Cistulli PA. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliances. JDSM. 2015; 2: 175–181.
- Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, et al. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013; 68: 91-96. PubMed: https://pubmed.ncbi.nlm.nih.gov/22993169/
- Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993; 147: 887-895. PubMed: https://pubmed.ncbi.nlm.nih.gov/8466125/
- Lee SD, Ju G, Kim JW, Yoon IY. Improvement of EEG slowing in OSAS after CPAP treatment. J Psychosom Res. 2012; 73: 126-131. PubMed: https://pubmed.ncbi.nlm.nih.gov/22789416/
- Wang G, Chen M, Bian J, He B. Electroencephalogram spectral power analysis of obstructive sleep apnea syndrome patients before and during continuous positive airway pressure therapy. Chin J Tuberculosis Respirat Dis. 2002; 25: 199: 203. PubMed: https://pubmed.ncbi.nlm.nih.gov/12133324/
- Grenèche J, Krieger J, Bertrand F, Ethardt C, Muzet A, et al. Effect of continuous positive airway pressure treatment on the subsequent EEG spectral power and sleepiness over sustained wakefulness in patients with obstructive sleep apnea-hypopnea syndrome. Clin Neurophysiol. 2011; 122: 958-965. PubMed: https://pubmed.ncbi.nlm.nih.gov/20889373/
- Puskás S, Kozák N, Sulina D, Csiba L, Magyar MT. Quantitative EEG in obstructive sleep apnea syndrome: a review of the literature. Rev Neurosci. 2017; 28: 265-270. PubMed: https://pubmed.ncbi.nlm.nih.gov/28099139/
- Montplaisir J, Bèdard MA, Richer F, Rouleau I. Neurobehavioral manifestations in obstructive sleep apnea syndrome before and after treatment with continuous positive airway pressure. Sleep. 1992; 15: 17–19. PubMed: https://pubmed.ncbi.nlm.nih.gov/1470802/
- Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, et al. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988; 94: 32–37. PubMed: https://pubmed.ncbi.nlm.nih.gov/3383654/
- Mathieu A, Mazza S, Petit D, Décary A, Massicotte-Marquez J, et al. Does age worsen EEG slowing and attention deficits in obstructive sleep apnea syndrome? Clin Neurophysiol. 2007; 118: 1538–1544. PubMed: https://pubmed.ncbi.nlm.nih.gov/17507290/
- Sforza E, Grandin S, Jouny C, Rochat T, Ibanez V. Is waking electroencephalographic activity a predictor of daytime sleepiness in sleeprelated breathing disorders? Eur Respir J. 2002; 19: 645–652. PubMed: https://pubmed.ncbi.nlm.nih.gov/11998993/
- Grenèche J, Krieger J, Erhardt C, Bonnefond A, Eschenlauer A, et al. EEG spectral power and sleepiness during 24 h of sustained wakefulness in patients with obstructive sleep apnea syndrome. Clin Neurophysiol. 2008; 119: 418–428. PubMed: https://pubmed.ncbi.nlm.nih.gov/18077207/
- Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000; 101: 523–529. PubMed: https://pubmed.ncbi.nlm.nih.gov/11113301/
- D’Rozario AL, Kim JW, Wong KK, Bartlett DJ, Marshall NS, et al. A new EEG biomarker of neurobehavioural impairment and sleepiness in sleep apnea patients and controls during extended wakefulness. Clin Neurophysiol. 2013; 124: 1605-1614. PubMed: https://pubmed.ncbi.nlm.nih.gov/23562656/
- Wang D, Piper AJ, Yee BJ, Wong KK, Kim JW, et al. Hypercapnia is a key correlate of EEG activation and daytime sleepiness in hypercapnic sleep disordered breathing patients. J Clin Sleep Med. 2014; 10: 517-522. PubMed: https://pubmed.ncbi.nlm.nih.gov/24910553/
- Grenèche J, Saremi M, Erhardt C, Hoeft A, Eschenlauer A, et al. Severity of obstructive sleep apnoea/hypopnoea syndrome and subsequent waking EEG spectral power. Eur Respir J. 2008; 32: 705–709. PubMed: https://pubmed.ncbi.nlm.nih.gov/18757699/
- Hayakawa T, Terashima M, Kayukawa Y, Ohta T, Okada T. Changes in cerebral oxygenation and hemodynamics during obstructive sleep apneas. Chest. 1996; 109: 916–921. PubMed: https://pubmed.ncbi.nlm.nih.gov/8635370/
- Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, et al. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015; 3: 404-414. PubMed: https://pubmed.ncbi.nlm.nih.gov/25887982/
- D’Rozario AL, Cross NE, Vakulin A, Bartlett DJ, Wong KKH, et al. Quantitative Electroencephalogram Measures in Adult Obstructive Sleep Apnea -Potential Biomarkers of Neurobehavioural Functioning. Sleep Med Rev. 2017; 36: 29-42.
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: Towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002; 11: 1-16. PubMed: https://pubmed.ncbi.nlm.nih.gov/11869421/
- Kraaier V, Van Huffelen AC, Wieneke GH. Quantitative EEG changes due to hypobaric hypoxia in normal subjects. Electroencephalogr Clin Neurophysiol. 1988; 69: 303–312. PubMed: https://pubmed.ncbi.nlm.nih.gov/2450729/
- Ozaki H, Watanabe S, Suzuki H. Topographic EEG changes due to hypobaric hypoxia at simulated high altitude. Electroencephalogr Clin Neurophysiol. 1995; 94: 349–356. PubMed: https://pubmed.ncbi.nlm.nih.gov/7774521/
- Wang D, Yee BJ, Wong KK, Kim JW, Dijk DJ, et al. Comparing the effect of hypercapnia and hypoxia on the electroencephalogram during wakefulness. Clin Neurophysiol. 2015; 126: 103-109. PubMed: https://pubmed.ncbi.nlm.nih.gov/24875233/
- Wang D, Thomas RJ, Yee BJ, Grunstein RR. Hypercapnia Is More Important Than Hypoxia in the Neuro- Outcomes of Sleep-Disordered Breathing. J Appl Physiol. 2016; 120: 1484–1486. PubMed: https://pubmed.ncbi.nlm.nih.gov/26869712/
- Harper RM, Kumar R, Ogren JA, Macey PM. Sleep-disordered breathing: effects on brain structure and function. Respir Physiol Neurobiol. 2013; 188: 383-391. PubMed: https://pubmed.ncbi.nlm.nih.gov/23643610/
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, et al. Brain structural changes in obstructive sleep apnea. Sleep. 2008; 31: 967-977. PubMed: https://pubmed.ncbi.nlm.nih.gov/18652092/
- Alchanatis M, Deligiorgis N, Zias N, Amfilochiou A, Gotsis E, et al. Frontal brain lobe impairment in obstructive sleep apnoea: a proton MR spectroscopy study. Eur Respir J. 2004; 24: 980–986. PubMed: https://pubmed.ncbi.nlm.nih.gov/15572542/
- Yaouhi K, Bertran F, Clochon P, Mezenge F, Denise P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009; 18: 36–48. PubMed: https://pubmed.ncbi.nlm.nih.gov/19250174/
- Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011; 183: 1419–1426. PubMed: https://pubmed.ncbi.nlm.nih.gov/21037021/
- Castronovo V, Canessa N, Strambi LF, Aloia MS, Consonni M, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep. 2009; 32: 1161–1172. PubMed: https://pubmed.ncbi.nlm.nih.gov/19750921/
- Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Oral appliance therapy versus nasal continuous positive airway pressure in obstructive sleep apnea: a randomized, placebo-controlled trial. Respiration. 2011; 81: 411–419. PubMed: https://pubmed.ncbi.nlm.nih.gov/20962502/
- Anandam A, Patil M, Akinnusi M, Jaoude P, El-Solh AA. Cardiovascular mortality in obstructive sleep apnea treated with continuous positive airway pressure or oral appliance: an observational study. Respirology. 2013; 18: 1184-1190. PubMed: https://pubmed.ncbi.nlm.nih.gov/23731062/
- Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004; 27: 934-941. PubMed: https://pubmed.ncbi.nlm.nih.gov/15453552/
- Otsuka R, Ribeiro de Almeida F, Lowe AA, Linden W, Ryan F. The effect of oral appliance therapy on blood pressure in patients with obstructive sleep apnea. Sleep Breath. 2006; 10: 29-36. PubMed: https://pubmed.ncbi.nlm.nih.gov/16391938/
- Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, et al. The effects of 1year treatment with a Herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007; 131: 740-749. PubMed: https://pubmed.ncbi.nlm.nih.gov/17356088/
- Trzepizur W, Gagnadoux F, Abraham P, Rousseau P, Meslier N, et al. Microvascular endothelial function in obstructive sleep apnea: Impact of continuous positive airway pressure and mandibular advancement. Sleep Med. 2009; 10: 746-752. PubMed: https://pubmed.ncbi.nlm.nih.gov/19147401/
- White DP, Shafazand S. Mandibular advancement device vs CPAP in the treatment of obstructive sleep apnea: are they equally effective in short term health outcomes? J Clin Sleep Med. 2013; 9: 971-972. PubMed: https://pubmed.ncbi.nlm.nih.gov/23997711/
- Lee CH, Mo JH, Choi IJ, Lee HJ, Seo BS, et al. The Mandibular Advancement Device and Patient Selection in the Treatment of Obstructive Sleep Apnea. Arch Otolaryngol Head Neck Surg. 2009; 135: 439-444. PubMed: https://pubmed.ncbi.nlm.nih.gov/19451462/
- Almeida FR, Bansback N. Long-Term Effectiveness of Oral Appliance versus CPAP Therapy and the Emerging Importance of Understanding Patient Preferences. Sleep. 2013: 36: 1271-1272. PubMed: https://pubmed.ncbi.nlm.nih.gov/23997356/
- Qaseem A, Holty JE, Owens DK, Dallas P, Starkey M, et al. Management of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2013; 159: 471-483. PubMed: https://pubmed.ncbi.nlm.nih.gov/24061345/
- Strohl KP, Brown DB, Collop N, George C, Grunstein R, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013; 187: 1259-1266. PubMed: https://pubmed.ncbi.nlm.nih.gov/23725615/
- Weaver TE. Don’t start celebrating—CPAP adherence remains a problem. J Clin Sleep Med. 2013; 9: 551-552. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3659374/
- Clark GT, Nakand M. Dental appliances for the treatment of obstructive sleep apnea. J Am Dent Ass. 1989; 118: 611-619.
- George PT. Treatment of snoring and obstructive sleep apnea with a dental device. Gen Dent. 1993; 41: 294-298. PubMed: https://pubmed.ncbi.nlm.nih.gov/8262341/
- Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001; 163: 1457-1461. PubMed: https://pubmed.ncbi.nlm.nih.gov/11371418/