More Information
Submitted: November 28, 2020 | Approved: December 29, 2020 | Published: December 30, 2020
How to cite this article: Gunduz ZB, Uygun T. High suspicion of paradoxical embolism due to atrial septal Defect: A rare cause of ischemic stroke. J Neurosci Neurol Disord. 2020; 4: 084-087.
DOI: 10.29328/journal.jnnd.1001040
ORCiD: orcid.org/0000-0002-6421-1857
Copyright License: © 2020 Gunduz ZB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Atrial septal defect; Paradoxical embolism; Ischemic stroke; Congenital heart disease; Cerebrovasculer disease
High suspicion of paradoxical embolism due to atrial septal Defect: A rare cause of ischemic stroke
Zahide Betul Gunduz1* and Turgut Uygun2
1Assistant Professor, Department of Neurology, University of Health Sciences Konya Education and Research Hospital, Turkey
2Department of Cardiology, University of Health Sciences Konya Education and Research Hospital, Turkey
*Address for Correspondence: Dr. Zahide Betül Gunduz, Assistant Professor, Department of Neurology, University of Health Sciences, Konya Education and Research Hospital, Ayanbey Mah. Yeni Meram Cad. No: 97, Meram, Konya, Turkey, Tel: 0090 5326208182; Fax: 0090 332 2210000; Email: [email protected]
Atrial septal defect (ASD) is common among adult congenital heart diseases but rarely causes paradoxical cerebral embolism. By sharing the ASD diagnosed after the first ischemic stroke attack at the age of 49 and a case of paradoxical cerebral embolism developing accordingly, we aimed to draw attention to the necessity of detailed cardiac examination in patients with cryptogenic stroke.
Stroke is one of the leading causes of morbidity and mortality worldwide. One of every four stroke patients experience a stroke in the following periods [1].
Cryptogenic stroke is defined as a symptomatic stroke where a possible cause is not identified. In order to make this diagnosis, it is essential to review and exclude all risk factors involved in etiology. It accounts for 30% - 40% of all ischemic strokes and is more common among young adults. When screened with appropriate examinations, the probability of revealing cardiac pathologies that play a role in etiology increases in this patient group [2].
Atrial septal defect (ASD) is common among adult congenital heart diseases, but it is rare to cause paradoxical embolism in the central nervous system. Paradoxical embolism is when the emboli originating from the venous system finds an abnormal gap between the right and left heart, passes into the systemic arterial circulation and then causes systemic embolic events [3]. Review of ASD in differential diagnosis in patients evaluated as cryptogenic stroke is important in terms of reducing the risk of recurrence, as it will change treatment planning.
A 49-year-old woman was evaluated with the complaints of unconsciousness and right-sided weakness in the emergency room. There was no known systemic disease or chronic drug use history. The patient, who was reported to be healthy for the last 6 hours ago, later slept, and found unconscious on the ground about a wheezing sound about 1 hour ago. There was no contraction, lock in teeth, and incontinence, but she did not respond and looked blank. She was brought to the hospital noticing that she had a bent mouth and weakness on her right side.
On neurological examination, she was conscious and anxious, and the cooperative motor could not be established due to marked mixed aphasia. Central type facial paralysis was present on the right. Her right side was hemiplegic and the base skin reflex extensor on the right.
ECG was in normal sinus rhythm and arterial blood pressure was 163/70 mmHg. No abnormalities were found in laboratory findings.
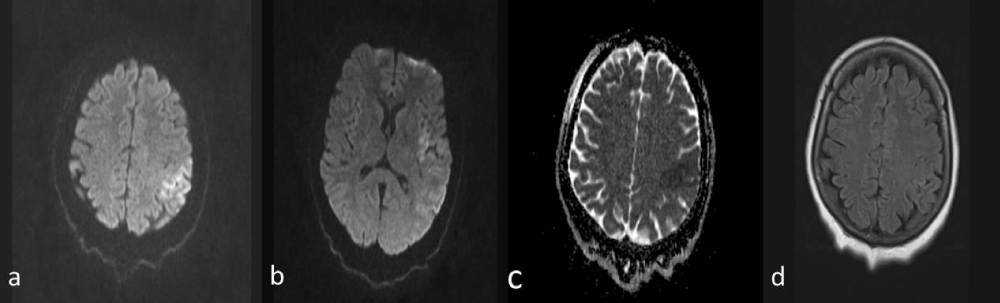
No pathology was detected in compute tomography (CT). Diffusion magnetic resonance imaging (MRI) showed diffuse restriction (Figure 1a,b) compatible with large-sized acute ischemia located in the left temporal lobe and insular cortex, frontoparietal, to the vertex level (Figure 1a,b), and the hypointense response in apparent diffusion coefficient (ADC) (Figure 1c). When the acute ischemic area was not observed in the flair sequence (Figure 1d), the patient was found to be suitable for intravenous thrombolytic therapy and taken to the stroke unit.
Figure 1: a,b) Diffusion magnetic resonance imaging (MRI); Diffusion restriction compatible with acute ischemia in the area supplied by the left middle cerebral artery. c) Hypointense in apparent diffusion coefficient (ADC). D) Flair sequence is normal.
The patient weighing 115 kg was started to complete 81 grams of intravenous infusion in one hour, following 9 grams of intravenous push in alteplase. When the infusion was completed, a great improvement was observed in the neurological deficit, muscle strength was 2-3/5 in the upper right, 4/5 in the lower right, while the aphasia remained in the form of word finding difficulties, the understanding was preserved. At the end of 24 hours, muscle strength was 4/5 in the right upper extremity and 4-5/5 in the lower extremity, and the control CT was evaluated as normal, antiaggregant therapy was applied (acetylsalicylic acid 100 mg/day + clopidogrel 75 mg/day). The patient, who became normotensive without using any antihypertensive therapy within hours, and her blood pressure was regulated during her follow-up.
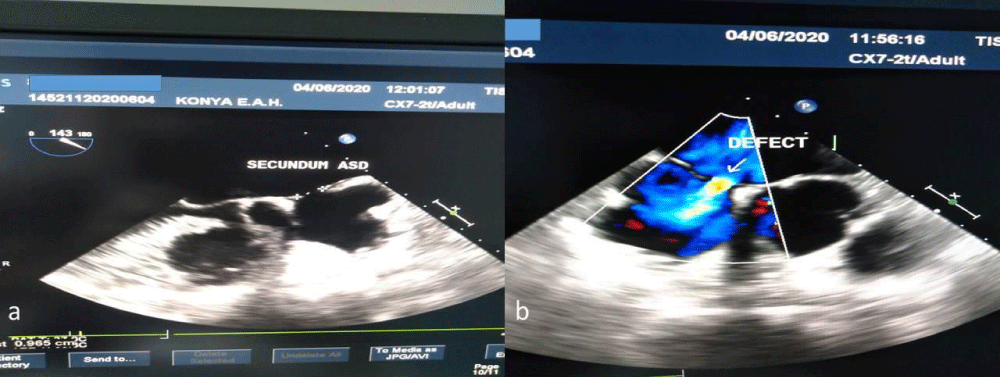
Neither CT angiography nor transthoracic echocardiography (TTE) and electrocardiography (ECG) holter revealed pathological findings in etiology. In transesophageal echocardiography (TEE), the intraatrial septum was aneurysmatic and there was a secundum ASD with an 8 mm diameter left right passage (Figure 2a,b). Defect sizes were visualized in the 2D evaluation on TEE, a clear left-right transition was observed with Color Doppler. Additional modality was not required. The patient stated that she had been operated 2 years ago with a diagnosis of deep vein thrombosis in the left lower extremity, was discontinued after using 6 months of oral anticoagulant, and had complaints of swelling and pain in the left leg intermittently for the last 15 days, but her complaints disappeared after suffering a stroke. In the lower extremity venous doppler ultrasonography (USG), in addition to the advanced tortiosity and enlargement in the lateral branches of the bilateral vena safena magna, medical treatment was recommended by cardiovascular surgery with the diagnosis of venous insufficiency. With the effect of thrombolytic therapy, deep vein thrombosis was thought to disappear. Accompanied by anamnesis, clinical and radiological findings, the table was evaluated as a paradoxical cerebral embolism developed after deep venous thrombosis and ASD association. Dual antiaggregant treatment was discontinued and low molecular weight heparin treatment was initiated. The patient, whose general condition was stable, was discharged without any neurological sequela and was directed to the advanced healthcare institution to plan ASD repair.
Figure 2: Transesophageal echocardiography (TEE). a) Secundum asd, b) Defect.
Stroke is one of the leading causes of morbidity and mortality worldwide. One of every four stroke patients experience a stroke in the following periods [1]. For this reason, the neurologist who encounters the stroke patient, while arranging the hyperacute period treatment on the one hand, on the other hand, targets the appropriate secondary prophylaxis by completing the examinations for the etiology completely.
No etiological cause can be revealed in a third of all ischemic strokes. Undiagnosed paroxysmal atrial fibrillation, patent foramen ovale (PFO) related paradoxical embolism, substenotic atherosclerosis, and other cardiac pathologies are likely to play a role in this group, called cryptogenic stroke [2]. For this reason, it is important to review all detailed investigations before diagnosing cryptogenic stroke in order to direct secondary prophylaxis
The term paradoxical embolism was first coined by Cohnheim in a case of middle meningeal artery ischemia in 1877 to describe a condition in which venous system-derived emboli pass through an abnormal communication between the right and left heart, entering the systemic arterial circulation, leading to subsequent systemic embolic events [3]. In recent years, the opinion that paradoxical embolism can be a potential source of cryptogenic stroke, especially in young patients, has gained weight. Although most cases of paradoxical embolism are associated with PFO, more rarely cases of paradoxical embolism associated with atrial septal defect (ASD), pulmonary arteriovenous malformation and other intracardiac septal defects have been reported [4].
In the triad defined for the diagnosis of paradoxical embolism; The presence of a proven systemic arterial embolism without an embolism originating from the left heart, the detection of a source of embolism in the venous system, and a pressure gradient with a right-to-left shunt and abnormal communication between the venous and arterial systems are required [4]. Although our patient had complaints consistent with deep vein thrombosis that started 15 days ago in the left leg, it was learned that her complaint completely disappeared after intravenous thrombolytic therapy. The presence of venous thrombus could not be detected because doppler ultrasonography was performed after thrombolytic therapy.
Although the relationship between PFO and cryptogenic stroke is the most discussed cardiac pathology, it is not yet clear whether the relationship with stroke is causal, and if there is a causal relationship, through which mechanism it is effective. However, the presence of PFO in 267 patients in a study in which 581 patients with ischemic stroke, considered cryptogenic, between the ages of 18-55 were examined, supports the possibility of a causal relationship [5].
In a study examining the relation of paradoxical embolism with ASD, paradoxical embolism was found in 20 (14%) of 329 patients with ASD, and it was revealed that these patients were younger in age and had smaller defects compared to the group without patioxal embolism. These findings indicate that the prevalence of paradoxical embolism in adult patients with ASD is higher than expected and this diagnosis should be reviewed in patients with cryptogenic stroke [6].
ASD is the third most common type of congenital heart disease, with an estimated incidence of 56 per 100,000 live births. There are three main types of ASD: ostium secundum, ostium primum and sinus venosus defect. Women make up 65% - 75% of patients with secundum ASD, but the gender distribution is equal for the other subtypes. Ostium secundum defect, the most common subtype, is located in the fossa ovalis region and is considered to be the true defect of the atrial septum. It does not combine with other structures and is the most frequently associated subtype of ASD with paradoxical ischemic embolism [7].
The clinical manifestations of ASD differ in the pediatric population and adults. It is mostly asymptomatic in childhood and is diagnosed following the recognition of a heart murmur during routine controls or by cardiac echocardiographic scanning in the neonatal period. If the defect is smaller than 6 mm, spontaneous closure can be expected in the neonatal period or pediatric period. The operation is usually planned according to the body size of the children before the primary school period when the mortality rate is low. In adults, ASD is usually symptomatic. It can be diagnosed as a result of palpitations, arrhythmias, or congestive heart failure [8].
The oldest known technique for repairing atrial septal defects is surgical closure. In recent years, transcatheter closure and treatment options are increasingly used. Although comparative studies evaluating the long-term results of the transcatheter technique are limited, preliminary data reveal its advantage in terms of significantly fewer complications and shorter hospital stay than reported for surgical repair [9].
Although the reports of transcatheter closure of ASD in pediatric or young adults have been intriguing over the last two decades, the data on the elderly ASD population are not clear. The anxiety that comorbid conditions such as essential hypertension, arrhythmia and respiratory distress may adversely affect the procedure to be applied, or the fact that the physiological adaptation that has improved in response to the congenital defect has decreased the need for closure may explain the limitation of data in the elderly generation (8). Although tolerance that develops over time shadows the symptomatic findings of the disease, it does not reduce the development of systemic complications. It is known that 11% of patients with ASD over the age of 40 are diagnosed after a serious clinical picture such as cerebral or pulmonary embolism [8].
Atrial arrhythmias are the most common comorbidities in adult patients with ASD. One third of patients over 60 years of age with ASD or half of patients over 70 years of age are complicated by atrial fibrillations [8]. In 9 elderly patients (mean age 68.1) with ASD and atrial fibrillation, it was reported that the closure of the defect with transcatheterization slowed down the rate of atrial fibrillation, cardiac functional recovery was observed, and hemodynamic and thromboembolic complications were not observed during the follow-up period (average 10.6 months) [10]. It is known that atrial fibrillation plays a role primarily in the etiology of cardioembolic stroke with embolism originating from the left atrial appendage thrombus. In conclusion, it should be kept in mind that closure of the defect does not prevent cardioembolic ischemia due to atrial fibrillation, although it prevents paradoxical embolism in patients with ASD and atrial fibrillation [11].
If a patient has a putative paradoxical cerebral embolism, surgery or transcatheter closure is recommended, even if the defect is anatomically small and there is no sign of cardiac overload [11]. While early closure of children and adolescents with ASD reduces the risk of tachyarrhythmia development later in life, there is no reduction in the risk of tachyarrhythmia in patients after closure in adulthood. Close follow-up of patients with ASD in terms of atrial fibrillation both before and after the defect is closed, and the use of effective anticoagulant therapy when detected may reduce the risk of cardioembolic ischemic stroke. It is known that long-term rhythm monitoring is more effective than short-term recording in the detection of paroxysmal atrial fibrillation [11]. Paroxysmal atrial fibrillation was not observed in the holter ECG monitoring of our patient, but close follow-up was planned during the controls.
One of the clear limiting factors in thrombolytic therapy is time. The period of time that allows treatment from the onset of the symptom is limited to 4.5 hours. If the onset time cannot be determined precisely, the last time the patient is normally seen is considered the “start time”. Failure to determine the starting time constitutes one fourth of the reasons for not giving thrombolytic therapy. The fact that about half of them have strokes during waking up is interpreted as the patients awaken during the stroke [12]. Considering this ratio, it has come to the fore to activate various parameters in order for this patient group to benefit from thrombolytic therapy.
The prevailing opinion is that thrombolytic treatment can be decided on the basis of imaging data in cases whose onset time cannot be determined exactly and exceeds 4.5 hours [12]. Diffusion MRI and FLAIR incompatibility, that is, the absence of the clear response of the acute ischemia site seen in diffusion in FLAIR, is one of these imaging data. We found our patient, who was healthy 6 hours ago and whose stroke onset time was not known, suitable for intravenous thrombolytic therapy, since no lesion was observed in the FLAIR sequence.
Although treatment options such as intravenous thrombolytic therapy or thrombectomy given to patients in the hyperacute period significantly reduce the mortality and morbidity rates in stroke patients, prophylactic treatment for the etiology is insufficient unless the risk of recurrence is reduced.
For this reason, it is of vital importance to review all differential examinations in cryptogenic strokes and to reveal the etiological pathologies that may change the treatment planning. Considering ASD in the differential diagnosis of strokes whose etiology has not been established is important in terms of changing the treatment protocol.
Ethics
Informed Consent: Informed consent was given by the patient.
- Esenwa C, Gutierrez J. Secondary stroke prevention: challenges and solutions. Vasc Health Risk Manag. 2015; 11: 437-450. PubMed: https://pubmed.ncbi.nlm.nih.gov/26300647/
- Yaghi S, Bernstein RA, Passman R, Okin PM, Furie KL, et al. Cryptogenic Stroke: Research and Practice. Circ Res. 2017; 120: 527-540. PubMed: https://pubmed.ncbi.nlm.nih.gov/28154102/
- Lippmann H, Rafferty T. Patent foramen ovale and paradoxical embolization: a historical perspective. Yale J Biol Med. 1993; 661: 11-17. PubMed: https://pubmed.ncbi.nlm.nih.gov/8256459/
- Fang ZF, Tang L, Zhou SH. Ischemic stroke caused by paradoxical embolism after an unsuccessful transcatheter atrial septal defect closure procedure: a word of caution. Pediatr Cardiol. 2012; 33: 366-369. PubMed: https://pubmed.ncbi.nlm.nih.gov/22120514/
- Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002; 333: 706-711. PubMed: https://pubmed.ncbi.nlm.nih.gov/11872892/
- Bannan A, Shen R, Silvestry FE, Herrmann HC. Characteristics of adult patients with atrial septal defects presenting with paradoxical embolism. Catheter Cardiovasc Interv. 2009; 74: 1066-1069. PubMed: https://pubmed.ncbi.nlm.nih.gov/19670302/
- Leppert M, Poisson SN, Carroll JD. Atrial Septal Defects and Cardioembolic Strokes. Cardiol Clin. 2016; 34: 225-230. PubMed: https://pubmed.ncbi.nlm.nih.gov/27150170/
- Akagi T. Current concept of transcatheter closure of atrial septal defect in adults. J Cardiol. 2015; 65: 17-25. PubMed: https://pubmed.ncbi.nlm.nih.gov/25308548/
- Moake L, Ramaciotti C. Atrial septal defect treatment options. AACN Clin Issues. 2005; 16: 252-266. PubMed: https://pubmed.ncbi.nlm.nih.gov/15876892/
- Taniguchi M, Akagi T, Ohtsuki S, Okamoto Y, Tanabe Y, et al. Transcatheter closure of atrial septal defect in elderly patients with permanent atrial fibrillation. Catheter Cardiovasc Interv. 2009; 73: 682-686. PubMed: https://pubmed.ncbi.nlm.nih.gov/19133674/
- Leppert M, Poisson SN, Carroll JD. Atrial Septal Defects and Cardioembolic Strokes. Cardiol Clin. 2016; 34: 225-230. PubMed: https://pubmed.ncbi.nlm.nih.gov/27150170/
- Topcuoglu MA, Arsava EM, Ozdemir AO, Gürkaş E, Örken DN, et al. Intravenous Thrombolytic Therapy in Acute Stroke: Problems and Solutions. Turk J Neurol 2017; 23: 162-175.