More Information
Submitted: 22 April 2020 | Approved: 25 May 2020 | Published: 26 May 2020
How to cite this article: Dutta R. PISA Syndrome-Orthopedic manifestation of a neurological disease? J Neurosci Neurol Disord. 2020; 4: 038-044.
DOI: 10.29328/journal.jnnd.1001032
ORCiD: orcid.org/0000-0002-6129-1038
Copyright License: © 2020 Dutta R. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: PISA; Syndrome; Pleurothotonus; Neurology; Neurodegenerative diseases
Abbreviations: AD: Alzheimer’s Disease; DLB: Dementia with Lewy Bodies; HD: Huntington’s Disease; SSPE: Subacute Sclerosing Panencephalitis; SD: Standard Deviation; COMT: Catechol-O-Methyltransferase; MSA-P: Multiple System Atrophy- Parkinsonian type
PISA Syndrome-Orthopedic manifestation of a neurological disease?
Rajib Dutta*
MD, Neurology, India
*Address for Correspondence: Rajib Dutta, MD, Neurology, India, Email: rajibdutta808@gmail.com
Pleurothotonus, commonly known as Pisa Syndrome (PS), is a rare neurological disorder characterized by lateral bending of the trunk with a tendency to lean to one side. This is typically mobile and resolves in supine position. It often presents as an incapacitating symptom of underlying neurodegenerative conditions like Parkinson’s disease, alzheimer’s disease, multisystem atrophy, dementia with Lewy bodies, progressive supranuclear palsy and even subacute sclerosing panencephalitis. It is known to be associated with neuroleptics, dopaminergic agents, valproic acid and lithium. PS is also seen in neurosurgical disorders like subdural hematoma, normotensive hydrocephalus, or as a late complication of pallidotomy in patients with PD. It can present either as an acute emergency or can develop gradually over time.PS tend to happen in coronal plane and can be controlled and managed if diagnosed in early stage. However, a chronic form known as “camptocormia” occurs often in a combined fashion with anteroposterior flexion which can improve to some extent, remain stable or even get worse. Pathophysiologic mechanism is not completely understood. This review will discuss all the updated literatures published in PS in terms of prevalence, pathophysiology, clinical manifestation, and treatment modalities.
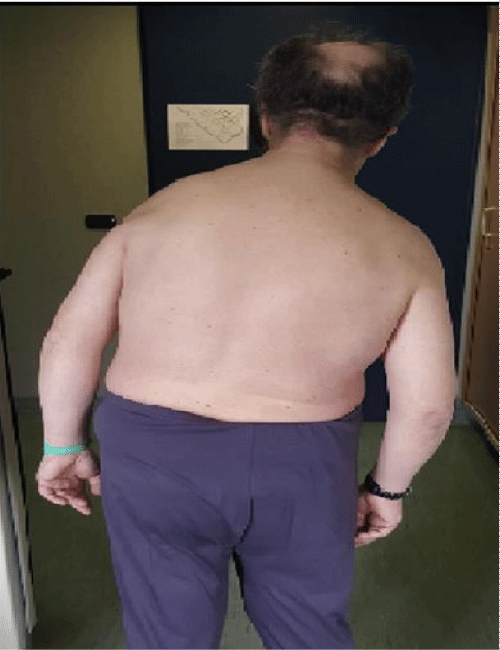
Pisa syndrome (PS) is a postural deformity which clinically presents as a marked lateral flexion of the trunk with a bending degree greater than 10° or 15°, typically mobile in nature and resolves at supine position. It is characterized by dystonia, and abnormal and sustained involuntary muscle contraction which may cause twisting or jerking movements of the body or a body part [1-3,17] (Figure 1). Ekbom, et al. originally described this entity which was then associated with butyrophenones [4]. It has been described in Parkinson’s disease (PD) either related to disease itself or secondary to levodopa or COMT inhibitors [9], atypical parkinsonism [5] and other neurodegenerative disorders like AD [6], DLB [7], HD [8], SSPE [10]. PS is linked to almost all dopaminergic agents, antipsychotics, valproate, benzodiazepines, lithium [11]. PS has been described in normotensive hydrocephalus [12,13], subdural hematoma [14], late complication of pallidotomy in a patient with PD [15] or even idiopathic [16].
Figure 1: Pisa syndrome. Note the leaning to the right.
Prevalence of PS is not well known because of the lack of clear diagnostic criteria. However, studies done previously reported that it may range from 2% to 90%. Tinazzi, et al. reported prevalence of 8.8% in PS patients with PD. The authors also reported that PS patients in their study were older, had lower body mass index, longer duration of disease in years, higher stages of disease, poorer quality of daily life, more frequent falls as well as occurrence of “veering gait” (the progressive deviation toward one side when patient walked forward and backward with eyes closed).The most frequently associated medical conditions were osteoporosis and arthrosis [3].
Prevalence rate of 8.3% (9.3% in women, 6.4% in men) was found in a Canadian study in which 133 patients were followed up for 2 days-3 months after typical neuroleptic exposure [18], contrary to a german study with psychiatric patients receiving antipsychotics where prevalence was reported 0.037% [19]. Risk factors associated in this study were females, old age, neuroleptic exposure and organic brain disorder. In a surveillance study which looked into the association of cholinesterase inhibitors and PS, mean (SD) age of 51 cases was 74.6 (8.2) and female: male ratio of 2:1 was reported [20]. In a single-center study from italy,26 patients were reported with lateral trunk deviation in the cohort of 1,400 patients with parkinsonism [21].Scoliosis is associated with PD and parkinsonism and can also be confused with PS [22-24].
Pathophysiology
The pathophysiology of PS is not clear. Accepted hypothesis till date are divided into central and peripheral. Central mechanisms mostly involve alteration in sensory-motor integration pathways, basal ganglia dysfunction secondary to cholinergic-dopaminergic imbalance whereas peripheral hypothesis is associated with anatomical changes in the musculoskeletal system [1,17,25,29,31].Cognitive processes and dysfunction also might be related to PS [26]. Altered visual-spatial functions and vertical perception deficits might represent a typical feature of PD patients with PS [26,27]. PS in PD patients may involve the nondopaminergic pathways as evident therapy-resistant symptom exists in this subset population [30].
Studies from animal models till date only suggests that there is a role of an asymmetric functioning of the nigrostriatal dopaminergic projections in PS [28].One study reported that, levodopa aggravated lateral flexion of neck and trunk post unilateral pallidotomy which may be a delayed phenomenon [32].Recently, role of vestibular function in maintaining posture was studied in parkinsonian patients. They found out unilateral peripheral vestibular hypofunction was present in all patients with lateral trunk flexion and the vestibular hypofunction was ipsilateral to the leaning side and contralateral to the most affected parkinsonian side in all the patients [33].
A dystonic activity might play a vital role in determining the bending ipsilaterally to PS and the contralateral excessive muscle activation which may be a compensatory mechanism [34]. An abnormal tonic hyperactivity on the side of the trunk’s deviation in abdominal oblique muscles was reported in PD patients with PS [35]. Frazzitta, et al. reported asymmetric ability to generate maximal voluntary force of the external oblique muscles supporting a central desynchronization of axial muscles as a significant contributor for the bending of the spine in erect position [36]. Degenerative spinal disease, soft tissue and muscle changes should not be forgotten while managing patients in clinical practice. Doherty, et al. in their study reported abnormal posture can be combination of degeneration of muscles and soft tissue secondary to dystonia which may be present from before or possibly a complex impairment of proprioceptive motor control in PD patients with PS [37].
Pathology
Hozumi and colleagues reported an autopsy case of a 62-year-old Japanese woman diagnosed as MSA-P with PS. This patient had a clinical history of lean towards her right side. Histopathological findings of brain showed neuronal loss and astrogliosis in the putamina more severe on the right side as compared to the left [38]. However a recent pathology report of a PD patient with PS found no significant basal ganglia asymmetry or brain stem involvement [39]. Peripheral pathology may be musculoskeletal in origin with myopathic alterations in paraspinal muscles [29].
Diagnosis
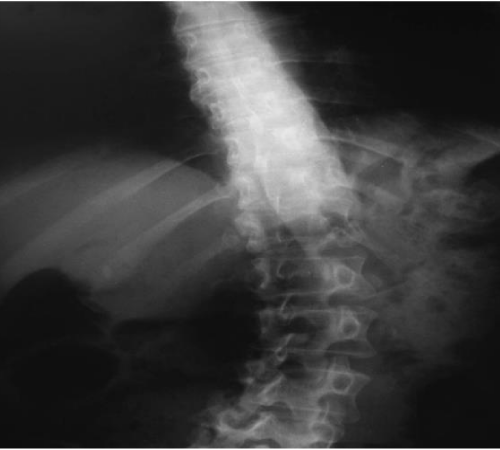
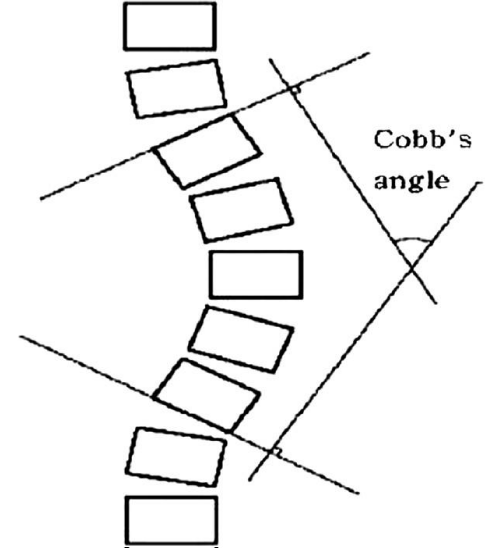
Diagnosis of PS requires at least 10° lateral flexion, which can be completely alleviated by passive mobilization or lying in a supine position [3,17]. Diagnosis of PS is based on the clinical evaluation of lateral displacement of the trunk, which is the first sign of postural misalignment usually reported by patients and their caregivers. Tracking can be done meticulously in day care clinical practice by goniometer, inclinometer or even by smartphone applications. X-rays in standing and supine positions is deemed necessary however standing is the most accurate method to assess the angle of curvature in the coronal and sagittal planes according to the Cobb angle to rule out other diagnoses [40,41] (Figures 2,3).
Figure 2: Anteroposterior radiograph of the PS patient exhibiting lean to the right.
Figure 3: Cobb’s angle.
| Table 1:Causes of PISA syndrome |
| Neurodegenerative diseases |
| Parkinson’s disease |
| Alzheimer’s disease |
| Lewy body dementia |
| Progressive supranuclear palsy |
| Huntington’s disease |
| Multiple system atrophy |
| Drugs |
| Levodopa combinations(levodopa/carbidopa,levodopa/benserazide,levodopa/carbidopa/entacapone) |
| Antipsychotics (typical and atypical) |
| Lithium |
| Cholinesterase inhibitors |
| Ergot derivative–pergolide |
| Nonergot derivatives–pramipexole and ropinirole |
| Monoamine oxidase-B inhibitor–rasagiline |
| Antiemetics |
| Selective serotonin reuptake inhibitors |
| Tricyclic antidepressants |
| Benzodiazepines |
| Valproate |
| Idiopathic form |
| Miscellaneous |
| Normal pressure hydrocephalus |
| Subdural hematoma |
| Subacute sclerosing panencephalitis |
| Table 2: Treatment modalities for PS |
| Pharmacological: High dose levodopa, anticholinergics, quetiapine, clozapine (cautiously), Istradefylline. |
| Botulinum Toxin (BoNT) |
| Surgical treatment:DBS,Spinal surgery |
| Orthotics |
| Rehabilitation |
Clinical manifestation
PS can develop in acute, subacute or chronic fashion [42-44]. PS patients generally lean towards one side while sitting, standing, and walking and also can have impaired perception of their vertical position awareness [17]. Recent exposure to antipsychotics and other medications may be the part of acute and subacute forms which benefits the most from anticholinergic treatment, however those with chronic exposure whose onset of the syndrome is insidious are less responsive to anticholinergic treatment [44-46]. This led Prahraj, et al. to classify PS into acute and tardive type [47]. However it is also reported that patients with chronic advanced type have a tendency to tilt to one side when sitting in a chair with subsequent lateral flexion during walking [48]. When the deformity worsens over time it can lead to dyspnea, low back pain [49], or unsteadiness leading to falls [50,51].
Differential diagnosis
PS can be differentiated from tardive dystonia [44], which is triggered by intake of dopamine receptor blockers such as antipsychotics for other causes, typically after chronic treatment of around 5 years, which may be persistent or even permanent even when the offending drug is discontinued [52]. Scoliosis is defined as the lateral curvature of the spine with a Cobb angle of 10 degrees or more in the coronal plane as measured on a radiograph [53]. This impairment in scoliosis is monoplaner and resolves when the primary abnormality is treated [54]. Muscle inflammatory disease like focal myositis [55], neuromuscular junction disorders specifically myasthenia gravis [56], and facioscapulohumeral dystrophy [57] should also be kept in mind while considering the diagnosis of PS.
Treatment
Pharmacological approaches: Drug-induced PS predominantly develops in women and older patients with organic brain changes [11,66]. In patients who has developed PS acute or subacutely it is mandatory for clinicians and neurologists to find out if there is any recent changes in medication dose or any new medication added to either modify or remove it respectively because PS in non-PD patients has been frequently related to the use of dopamine receptor blockers or cholinesterase inhibitors [63-65].
Axial symptoms and PS respond poorly to dopaminergic therapy, however other dopaminergic agents like levodopa in high dosage can be tried in patients where PS appears as a motor complication during the off periods [58,61]. Other agents which can be used in PS may include anticholinergics [16] and novel antipsychotics without interference with dopaminergic receptors like clozapine [59], quetiapine, however clozapine has been reported in literature to cause PS [60].
Clinicians should be very precautious when prescribing atypical antipsychotics because they can induce and aggravate PS [62]. Slower withdrawal of dopamine agonists might be another option as evidenced in patients with antecollis [17]. One recently published research reported that postural deformities caused by dopamine agonists generally improve less than two weeks after dopamine agonist withdrawal. Istradefylline which is used as an add-on treatment to levodopa/carbidopa in adults with PD experiencing “off “ episodes can be a potential therapeutic option in postural deformities like PS [83].
Botulinum toxin: Excessive muscular hyperactivity in PS can be treated with botulinum toxin (BoNT) [67]. In a blinded crossover study by Bonanni, et al. six patients treated with BoNT showed an improvement between 50% and 87.5%. In one patient, only subjective benefit was reported, while two patients did not report any benefit [21]. Injection in paraspinal muscles should be avoided, and top most priority should be given to infiltrate external oblique muscle [36]. Iliopsoas and the rectus abdominis were the most frequently used muscle for injection in study conducted by Tassorelli, et al. [68]. Dupeyron, et al. reported complete and 1 year lasting resolution of PS after BoNT injection in the quadratus lumborum muscle [69]. Two unique studies also found out BoNT is useful to enhance the effect of rehabilitation treatment in PS patients [68,70].
Orthotics: Spinal short segment decompression can be considered in appropriate candidates with spinal stenosis with radiculopathy or myelopathy as supported by a study on patients having PD with camptocormia [71].
Rehabilitation: Capecci, et al. conducted a single blind randomized clinical trial in which they applied postural rehabilitation for 4 weeks on 13 patients with PD and postural abnormalities including PS with 1-month follow-up. Six patients in the treatment group also had Kinesio taping strips applied to their trunk muscles. All treated patients showed a significant improvement in trunk posture in both the sagittal and coronal planes compared with baseline. Moreover, they showed an improvement in measures of gait and balance. They also concluded that combination of active posture correction and trunk movements, muscle stretching, and proprioceptive stimulation may usefully impact axial symptoms in PD. Repeated training was recommended to these patients to avoid waning of the effect. No differences were found between patients who received postural rehabilitation plus Kinesio taping bands compared with those receiving postural rehabilitation only [72].
One study also demonstrated significant improvements in axial posture and trunk mobility through the 4-week rehabilitation programme in patients with lateral trunk flexion in PD. However, the benefit was not sustained and diminished after a few months [73]. Frazzitta, et al. in their study also reported early rehabilitation before development of postural deformities in PD patients emphasizing specially on stretching exercises for the external oblique and paraspinal muscles [36]. Motor rehabilitation is an effective tool for PD patients with PS [68,70,74]. A structured exercise program to strengthen of contralateral paraspinal muscles may be a promising strategy [34].
Surgical treatment: Deep brain stimulation (DBS) of subthalmic nucleus (STN) does not have a good outcome in amelioration of PD axial symptoms, freezing of gait, falls, including posture and postural instability [75]. Stefani, et al. studied stimulation of pedunculopontine nucleus (PPN) in severe PD. They suggested patients with advanced PD, PPN-DBS associated with standard STN-DBS may be useful in improving gait and in optimizing the dopamine-mediated ON-state, particularly in those whose response to STN only DBS has deteriorated over time [76].
Shih, et al. reported improvement of gait and lean in PS patient with contralateral pedunculopontine stimulation [77]. Ricciardi, et al. reported about a case where stimulator electrodes were implanted in PPN ipsilateral to the side of the bend because of its role in promoting atonia. However, improvement did not sustain over time [78]. Spinal surgery may be useful in complex spinal deformities where conservative management has failed however selection procedure should be individualized and meticulous [79].
Postural deformities in PD is not uncommon in clinical practice but sometimes there can be an overlap of two conditions in the same patient like camptocormia and PS which makes situation for clinicians more difficult in terms of treatment. The pathophysiological mechanism may be same or at least closely related [44]. The etiology of this condition may be difficult to find as there is no consensus definition of the abnormalities in the coronal plane.
PS can be acute, subacute or can develop slowly with the evolvement of underlying disease. Acute and subacute forms may be dystonia like phenomenon which needs more understanding at this point of time [81]. Chronic changes may be changes induced over long time affecting muscle and bones [44,50]. More recent reports suggests the association between PS and specific cognitive alterations, implying a potential contribution of cortical and subcortical dysfunctions in the pathophysiology of PS [82]. Gradual withdrawal of drugs causing PS can be beneficial along with use of anticholinergics in patients exposed to antipsychotics due to any reason [46,80]. Other options may be dopaminergic agents like levodopa, BoNT, orthotics, rehabilitation and surgery in the form of DBS or spinal surgery.
PS is considered to be an overlooked condition with limited epidemiologic data in clinical practice. It is common yet not specific to patients with PD only. It has a significant impact on quality of life and daily functioning of an individual. A complex interplay between central basal ganglia dysfunction, together with proprioceptive disintegration and altered cognitive processing, is usually required for the development of PS. Physical symptoms like low back pain, dyspnea and fall may follow if not treated appropriately. Early recognition is must because it can be reversed at an acute or subacute stage. Future studies with an intention to gather enormous epidemiological data, understanding of pathophysiological mechanisms, consensus on diagnostic criteria followed by case to case based management is mandatory.
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. Special thanks to my supervisor Professor Dr. Huifang Shang who gave initial ideas and supported me through this research study. I would also like to thank Dr. Swatilekha Roy Sarkar for her valuable feedback on the manuscript and PubMed literature screening.
- Barone P, Santangelo G, Amboni M, Pellecchia MT, Vitale C. Pisa syndrome in Parkinson's disease and parkinsonism: clinical features, pathophysiology, and treatment. Lancet Neurol. 2016; 15: 1063–1074. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27571158
- Geroin C, Squintani G, Morini A, Donato F, Smania N, et al. Pisa syndrome in Parkinson's disease: electromyographic quantification of paraspinal and non-paraspinal muscle activity. Funct Neurol. 2017; 32: 143–151. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29042003
- Tinazzi M, Fasano A, Geroin C. Pisa syndrome in Parkinson disease: An observational multicenter Italian study.Neurology.2015; 85: 1769–1779. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26491088
- Ekbom K, Lindholm H, Ljungberg L. New dystonic syndrome associated with butyrophenone therapy. Z Neurol. 1972; 202: 94–103. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4115928
- Marsili L, Bologna M, Kojovic M, Berardelli A, Espay AJ, et al. Dystonia in atypical parkinsonian disorders. Parkinsonism Relat Disord. 2019; 66: 25–33. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31443953
- Woo KA, Yoo D, Ki CS, Lee JY. Spontaneous Pisa syndrome in a patient with early-ons, et al. zheimer's disease. Neurol Sci. 2019.
- Shinfuku M, Nakajima S, Uchida H, Watanabe K, Kocha H, et al. Pisa syndrome caused by an acetylcholinesterase inhibitor in a patient with dementia with Lewy bodies. Psychiatry Clin Neurosci. 2011; 65: 299. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21507139
- Salazar Z, Tschopp L, Calandra C, Micheli F. Pisa syndrome and parkinsonism secondary to valproic acid in Huntington's disease. Mov Disord. 2008; 23: 2430–2431. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18823027
- Solla P, Cannas A, Congia S, Floris G, Aste R, et al. Levodopa/carbidopa/entacapone-induced acute Pisa syndrome in a Parkinson's disease patient. J Neurol Sci. 2008; 275: 154–156. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18814889
- Pandey S, Tomar LR, Tater P. Pisa Syndrome in a Child With Subacute Sclerosing Panencephalitis. JAMA Neurol. 2018; 75: 255–256. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29297046
- Suzuki T, Matsuzaka H. Drug-induced Pisa syndrome (pleurothotonus): epidemiology and management. CNS Drugs.2002; 16: 165–174. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11888337
- Todisco M, Pozzi NG, Zangaglia R, Minafra B, Servello D, et al. Pisa syndrome in Idiopathic Normal Pressure Hydrocephalus. Parkinsonism Relat Disord. 2019; 66: 40–44. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31300263
- Leon-Sarmiento FE, Pradilla G, Del Rosario Zambrano M. Primary and Reversible Pisa Syndrome in Juvenile Normal Pressure Hydrocephalus. Acta Neuropsychiatr. 2013; 25: 57–60. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3686565/
- Marchione P, Spallone A, Valente M, Giannone C, De Angelis F, Meco G. Reversible Pisa syndrome associated to subdural haematoma: case-report. BMC Neurol. 2014; 14: 149. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25123109
- van deWarrenburg BP, Bhatia KP, Quinn NP. Pisa syndrome after unilateral pallidotomy in Parkinson's disease: an unrecognised, delayed adverse event? J Neurol Neurosurg Psychiatry. 2007; 78: 329–330. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2117655/
- Bhattacharya KF, Giannakikou I, Munroe N, Chaudhuri KR. Primary anticholinergic-responsive Pisa syndrome. Mov Disord. 2000; 15: 1285–1287. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11104229
- Doherty KM, van de Warrenburg BP, Peralta MC, Moriyama LS, Azulay JP, et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011; 10: 538-549. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21514890
- Yassa R, Nastase C, Cvejic J, Laberge G. The Pisa syndrome (or pleurothotonus): prevalence in a psychogeriatric population. Biol Psychiatry. 1991; 29: 942–945. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1675591
- Stübner S, Padberg F, Grohmann R, et al. Pisa syndrome (pleurothotonus): report of a multicenter drug safety surveillance project. J Clin Psychiatry. 2000; 61: 569–574. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10982199
- Zannas AS, Okuno Y, Doraiswamy PM. Cholinesterase inhibitors and Pisa syndrome: a pharmacovigilance study. Pharmacotherapy. 2014; 34: 272–278. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24127392
- Bonanni L, Thomas A, Varanese S, Scorrano V, Onofrj M. Botulinum toxin treatment of lateral axial dystonia in Parkinsonism. Mov Disord. 2007; 22: 2097–2103. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17685467
- Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006; 21: 1856–1863. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16941460
- Baik JS, Kim JY, Park JH, Han SW, Park JH, et al. Scoliosis in patients with Parkinson's disease. J Clin Neurol. 2009; 5: 91–94. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19587816
- Grimes JD, Hassan MN, Trent G, Halle D, Armstrong GW. Clinical and radiographic features of scoliosis in Parkinson's disease. Adv Neurol. 1987; 45: 353–355. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3825710
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015; 30: 1591–1601. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26474316
- Vitale C, Falco F, Trojano L, Erro R, Moccia M, et al. Neuropsychological correlates of Pisa syndrome in patients with Parkinson's disease. Acta Neurol Scand. 2016; 134: 101–107. https://www.ncbi.nlm.nih.gov/pubmed/26427765
- Huh YE, Kim K, Chung WH, Youn J, Kim S, et al. Pisa Syndrome in Parkinson's Disease: Pathogenic Roles of Verticality Perception Deficits. Sci Rep. 2018; 8: 1804. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29379091
- Castrioto A, Piscicelli C, Perennou D, Krack P, Debu B. The pathogenesis of Pisa syndrome in Parkinson’s disease. Mov Disord.2014; 29: 1100-1107. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24909134
- Tinazzi M, Geroin C, Gandolfi M, Smania N, Tamburin S, et al. Pisa syndrome in Parkinson's disease: An integrated approach from pathophysiology to management. Mov Disord. 2016; 31: 1785–1795. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27779784
- Vorovenci RJ, Biundo R, Antonini A. Therapy-resistant symptoms in Parkinson's disease. J Neural Transm (Vienna). 2016; 123: 19–30. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26410626
- Villarejo A, Camacho A, García-Ramos R, Moreno T, Penas M, et al. Cholinergic-dopaminergic imbalance in Pisa syndrome. Clin Neuropharmacol. 2003; 26: 119–121. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12782913
- Spanaki C, Zafeiris S, Plaitakis A. Levodopa-aggravated lateral flexion of the neck and trunk as a delayed phenomenon of unilateral pallidotomy.Mov Disord. 2010; 25: 655–656. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20131399
- Vitale C, Marcelli V, Furia T, Santangelo G, Cozzolino A, et al. Vestibular impairment and adaptive postural imbalance in parkinsonian patients with lateral trunk flexion. Mov Disord. 2011; 26: 1458–1463. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21465552
- Tinazzi M, Juergenson I, Squintani G, Vattemi G, Montemezzi S, et al. Pisa syndrome in Parkinson’s disease: an electrophysiological and imaging study.J Neurol. 2013; 260: 2138–2148. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23695587
- Tassorelli C, Furnari A, Buscone S, Alfonsi E, Pacchetti C, et al. Pisa syndrome in Parkinson’s disease: clinical, electromyographic, and radiological characterization.Mov Disord. 2012; 27: 227–235. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21997192
- Frazzitta G, Balbi P, Gotti F, Maestri R, Sabetta A, et al. Pisa syndrome in Parkinson’s disease: electromyographic aspects and implication for rehabilitation. Parkinsons Dis. 2015; 2015: 437190. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26682083
- Doherty KM, Davagnanam I, Molloy S, Silveira-Moriyama L, Lees AJ. Pisa syndrome in Parkinson’s disease: a mobile or fixed deformity? J Neurol Neurosurg Psychiatry. 2013; 84: 1400–1403. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23532719
- Hozumi I, Piao YS, Inuzuka T, Matsuyama Z, Yamada Y, et al. Marked asymmetry of putaminal pathology in an MSA-P patient with Pisa syndrome. Mov Disord. 2004; 19: 470–472. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15077247
- Solla P, Grau-Rivera O, Gelpi E, Marrosu F, Martí MJ. Pisa syndrome in a patient with pathologically confirmed Parkinson's disease. Neuropathol Appl Neurobiol. 2016; 42: 654–658. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26999006
- PubMed: Vrtovec T, Pernus F, Likar B. A review of methods for quantitative evaluation of spinal curvature. Eur Spine J. 2009; 18: 593–607. https://www.ncbi.nlm.nih.gov/pubmed/19247697
- Vrtovec T, Pernus F, Likar B. A review of methods for quantitative evaluation of axial vertebral rotation. Eur Spine J. 2009; 18: 1079–1090. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2899509/
- Cannas A, Solla P, Floris G, Tacconi P, Serra A, et al. Reversible Pisa syndrome in patients with Parkinson’s disease on dopaminergic therapy. J Neurol. 2009; 256: 390–395. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19319462
- Miletić V, Radić B, Relja M. Acute pisa syndrome as a neurological emergency. J Neuropsychiatry Clin Neurosci. 2015; 27: e159–e160. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25923868
- Yokochi F. Lateral flexion in Parkinson's disease and Pisa syndrome. J Neurol. 2006; 253 Suppl 7: VII17–VII20. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17131222
- Stübner S, Padberg F, Grohmann R, Hampel H, Hollweg M, et al. Pisa syndrome (pleurothotonus): report of a multicenter drug safety surveillance project. J Clin Psychiatry. 2000; 61: 569–574. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10982199
- Suzuki T, Koizumi J, Moroji T, Sakuma K, Hori M, et al. Clinical characteristics of the Pisa syndrome. Acta Psychiatr Scand. 1990; 82: 454–457.
- Praharaj SK, Arora M. Pisa syndrome: acute and tardive forms. South Med J. 2007; 100: 853–854.
- Martin JP. Curvature of the spine in post-encephalitic parkinsonism. J Neurol Neurosurg Psychiatry. 1965; 28: 395–400. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/5838474
- Geroin C, Gandolfi M, Bruno V, Smania N, Tinazzi M. Integrated Approach for Pain Management in Parkinson Disease. Curr Neurol Neurosci Rep. 2016; 16: 28. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26879763
- Di Matteo A, Fasano A, Squintani G, Ricciardi L, Bovi T, et al. Lateral trunk flexion in Parkinson's disease: EMG features disclose two different underlying pathophysiological mechanisms. J Neurol. 2011; 258: 740–745. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21079986
- Geroin C, Smania N, Schena F, Dimitrova E, Verzini E, et al. Does the Pisa syndrome affect postural control, balance, and gait in patients with Parkinson's disease? An observational cross-sectional study. Parkinsonism Relat Disord. 2015; 21: 736–741. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25940999
- Saifee TA, Edwards MJ. Tardive movement disorders: a practical approach. Pract Neurol. 2011; 11: 341–348.
- Schwab FJ, Smith VA, Biserni M, Gamez L, Farcy JP, et al. Adult scoliosis: a quantitative radiographic and clinical analysis. Spine (Phila Pa 1976). 2002; 27: 387–392. PubMed: https://pubmed.ncbi.nlm.nih.gov/11840105
- El-Hawary R, Chukwunyerenwa C. Update on evaluation and treatment of scoliosis. Pediatr Clin North Am. 2014; 61: 1223–1241. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25439021
- Wunderlich S, Csoti I, Reiners K, Günthner-Lengsfeld T, Schneider C, et al. Camptocormia in Parkinson's disease mimicked by focal myositis of the paraspinal muscles. Mov Disord. 2002; 17: 598–600. https://www.ncbi.nlm.nih.gov/pubmed/12112214
- Abboud H, Sivaraman I, Ontaneda D, Tavee J. Camptocormia and Pisa syndrome as manifestations of acute myasthenia gravis exacerbation. J Neurol Sci. 2015; 359: 8–10. https://www.ncbi.nlm.nih.gov/pubmed/26671078
- Doherty KM, Silveira-Moriyama L, Giladi N, Bhatia KP, Parton M, et al. Camptocormia: don’t forget muscle disease in the movement disorder clinic. J Neurol. 2012; 259: 1752–1754. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22349871
- Kim JS, Park JW, Chung SW, Kim YI, Kim HT, et al. Pisa syndrome as a motor complication of Parkinson's disease. Parkinsonism Relat Disord. 2007; 13: 126–128. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16731022
- Arora M, Praharaj SK, Sarkar S. Clozapine effective in olanzapine-induced Pisa syndrome. Ann Pharmacother. 2006; 40: 2273–2275. PubMed: https://pubmed.ncbi.nlm.nih.gov/17132806/
- Kurtz G, Kapfhammer HP, Peuker B. Pisa syndrome in clozapine therapy [in German]. Nervenarzt. 1993; 64: 742–746. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8278016
- Michel SF, Arias Carrión O, Correa TE, Alejandro PL, Micheli F. Pisa Syndrome. Clin Neuropharmacol. 2015; 38: 135–140. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26166239
- Walder A, Greil W, Baumann P. Drug-induced Pisa syndrome under quetiapine. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33: 1286–1287. https://www.ncbi.nlm.nih.gov/pubmed/19646500
- Kwak YT, Han IW, Baik J, Koo MS. Relation between cholinesterase inhibitor and Pisa syndrome. Lancet. 2000; 355: 2222. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10881902
- Srivanitchapoom P, Hallett M. Camptocormia in Parkinson's disease: definition, epidemiology, pathogenesis and treatment modalities. J Neurol Neurosurg Psychiatry. 2016; 87: 75–85. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25896683
- Zannas AS, Okuno Y, Doraiswamy PM. Cholinesterase inhibitors and Pisa syndrome: a pharmacovigilance study. Pharmacotherapy. 2014; 34: 272–278. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24127392
- Suzuki T, Matsuzaka H. Drug-induced Pisa syndrome (pleurothotonus): epidemiology and management. CNS Drugs. 2002; 16: 165–174. https://www.ncbi.nlm.nih.gov/pubmed/11888337
- Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004; 75: 951–957. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1739107/
- Tassorelli C, De Icco R, Alfonsi E, Bartolo M, Serrao M, et al. Botulinum toxin type A potentiates the effect of neuromotor rehabilitation of Pisa syndrome in Parkinson disease: a placebo controlled study. Parkinsonism Relat Disord. 2014; 20: 1140–1144. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25175601
- Dupeyron A, Viollet E, Coroian F, Gagnard C, Renard D, et al. Botulinum Toxin-A for treatment of Pisa syndrome: A new target muscle. Parkinsonism Relat Disord. 2015; 21: 669–670. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25899457
- Santamato A, Ranieri M, Panza F, Zoccolella S, Frisardi V, et al. Botulinum toxin type A and a rehabilitation program in the treatment of Pisa syndrome in Parkinson's disease. J Neurol. 2010; 257: 139–141. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19763384
- Upadhyaya CD, Starr PA, Mummaneni PV. Spinal deformity and Parkinson disease: a treatment algorithm. Neurosurg Focus. 2010; 28: E5. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20196652
- Capecci M, Serpicelli C, Fiorentini L, Censi G, Ferretti M, et al. Postural rehabilitation and Kinesio taping for axial postural disorders in Parkinson's disease. Arch Phys Med Rehabil. 2014; 95: 1067–1075. https://www.ncbi.nlm.nih.gov/pubmed/24508531
- Bartolo M, Serrao M, Tassorelli C, Don R, Ranavolo A, et al. Four-week trunk-specific rehabilitation treatment improves lateral trunk flexion in Parkinson's disease. Mov Disord. 2010; 25: 325–331. https://www.ncbi.nlm.nih.gov/pubmed/20131386
- Kataoka H, Ikeda M, Horikawa H, Ueno S. Reversible lateral trunk flexion treated with a rehabilitation program in a patient with Parkinson's disease. Parkinsonism Relat Disord. 2013; 19: 494–497. https://www.ncbi.nlm.nih.gov/pubmed/23274084
- Visser JE, Allum JH, Carpenter MG, Esselink RA, Speelman JD, et al. Subthalamic nucleus stimulation and levodopa-resistant postural instability in Parkinson's disease. J Neurol. 2008; 255: 205–210. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18274810
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain. 2007; 130: 1596–1607. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17251240
- Shih LC, Vanderhorst VG, Lozano AM, Hamani C, Moro E. Improvement of pisa syndrome with contralateral pedunculopontine stimulation. Mov Disord. 2013; 28: 555–556. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23389993
- Ricciardi L, Piano C, Bentivoglio AR, Fasano A. Long-term effects of pedunculopontine nucleus stimulation for Pisa syndrome. Parkinsonism Relat Disord. 2014; 20: 1445–1446. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25455696
- Ha Y, Oh JK, Smith JS, Ailon T, Fehlings MG, et al. Impact of Movement Disorders on Management of Spinal Deformity in the Elderly. Neurosurgery. 2015; 77 Suppl 4: S173–S185. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26378355
- Suzuki T, Hori T, Baba A, Abe S, Shiraishi H, et al. Effectiveness of anticholinergics and neuroleptic dose reduction on neuroleptic-induced pleurothotonus (the Pisa syndrome). J Clin Psychopharmacol. 1999; 19: 277–280. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10350039
- Fasano A, Di Matteo A, Vitale C, Squintani G, Ferigo L, et al. Reversible Pisa syndrome in patients with Parkinson's disease on rasagiline therapy. Mov Disord. 2011; 26: 2578–2580. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22170277
- Artusi CA, Montanaro E, Tuttobene S, Romagnolo A, Zibetti M, et al. Pisa Syndrome in Parkinson's Disease Is Associated With Specific Cognitive Alterations. Front Neurol. 2019; 10: 577. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31214112
- Fujioka S, Yoshida R, Nose K, Hayashi Y, Mishima T, et al. A new therapeutic strategy with istradefylline for postural deformities in Parkinson's disease. Neurol Neurochir Pol. 2019; 53: 291‐295. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31441493