More Information
Submitted: 08 January 2020 | Approved: 23 January 2020 | Published: 24 January 2020
How to cite this article: Luisetto M, Khan FA, Muhamad A, Mashori GR, Ahmadabadi BN, et al. Brain washing systems and other circulating factors in some neurological condition like Parkinson (Pd) and vascular and diabetic dementia: How dynamics- saturation of clearance can act on toxic molecule? J Neurosci Neurol Disord. 2020; 4: 001-013.
DOI: 10.29328/journal.jnnd.1001028
Copyright License: © 2020 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Brain glymphatic system; Brain washing system; Vascular dementia; PD; DA; Diabetic dementia; New therapeutic strategy; Toxicology approach; Clearance of wasting molecule; Kinetics; Saturation; Genetic; Depurative approach
Brain washing systems and other circulating factors in some neurological condition like Parkinson (Pd) and vascular and diabetic dementia: How dynamics- saturation of clearance can act on toxic molecule?
Mauro Luisetto1*, Farhan Ahmad Khan2, Akram Muhamad3, Ghulam Rasool Mashori4, Behzad Nili Ahmadabadi5 and Oleg Yurevich Latiyshev6
1Applied Pharmacologist, Ima Academy, Pharmacology and Natural Science, Italy
2Professor and Head, Department of Pharmacology, Government Medical College, Shahdol, MP, India
3Department of Eastern Medicine, Government College, Faisalabad University, Pakistan
4Department of Medical & Health Sciences for Woman, Peoples University of Medical and Health Sciences for Women, Pakistan
5Pharm D/PhD, Innovative Pharmaceutical Product Development Specialist, USA
6President of IMA Academy, Italy
*Address for Correspondence: Mauro Luisetto, Applied Pharmacologist, Ima Academy, Pharmacology and Natural Science, Italy, Email: [email protected]; [email protected]
Observing the epidemiology of some neurodegenerative disease is interesting to verify some similarity and also related advanced or non-advanced countries and related diet habits. There are relationship between this conditions and diet habits? Some neurological condition related neuro-degeneration can be related to a complex dynamic system like the glymphatic system and the brain vascular clearance. Failure in this system seem related to aggravates of some condition like PD or vascular or diabetic dementia. (Animal model). But what happen if this dynamic system is saturated? A deep investigation related the specific role in CNS make possible to search new innovative strategies. The social economic cost for the neurodegenerative disease is the right tool to new research.
Before to start this work is interesting to observe some epidemiologic data involved in Neruodegeneretaive conditions like PD and DA: Is possible to observe that there are different in the various part of the world (advanced vs. non advanced countries, north vs. south of the world), and also inside the same country. This make possible to consider the environmental factor relevant in this situations? Added to other Endogenous factors? Various factors are involved in some neurodegenerative condition (progress of the disease) and in recent time the functionality of some CNS systems are deeply investigate more than past. Different kind of neurological disease present different presentation of the neuronal damages: Since from dementia A to PD to vascular or other dementia. Change the neuron involved and change the toxic sustantia involved with accumation (relationship? cause or effect?). What is clear is the final neurotoxicity. The same vascular dysfunction are involved in some kind of DEMENTIA. Other factor to be considered is that many neurodegenerative condition present an accumulation of some cathabolic substantia.
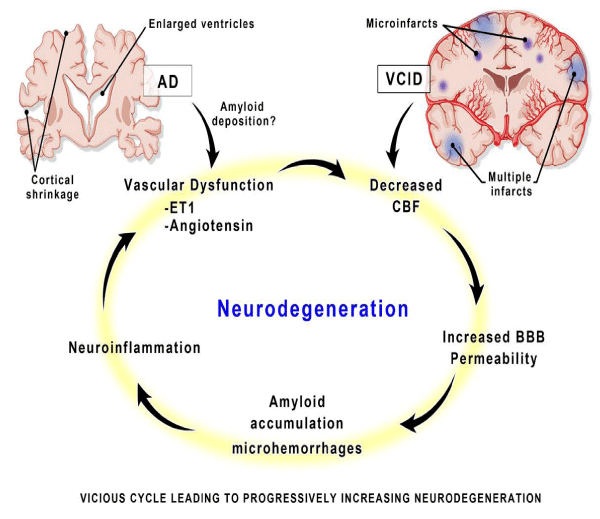
Although, Alzheimer disease (AD) and vascular cognitive impairment/dementia (VCID) may enter the cycle at different initiating points, the mediators of damage are similar and occur long before clinical symptoms present. BBB indicates blood-brain barrier; CBF, cerebral blood flow; and ET-1, endothelin 1. In diabetic dementia glicosilated product are responsible of the damages. (A product of metabolism). So to observe the system by which the brain is “DEPURED” can be interesting. The glymphatic system is a functional waste clearance pathway for the vertebrate (CNS). It consists of a para-arterial influx route way for cerebrospinal fluid to enter the brain parenchyma, coupled to a clearance mechanism for the removal of interstitial fluid and extra-cellular solutes from the interstitial compartments of the brain and spinal cord.
Exchange of solutes between CSF and ISF is driven primarily by arterial pulsation and regulated during sleep by the expansion and contraction of brain extra-cellular space. Clearance of soluble proteins, waste products, and excess extracellular fluid is accomplished through convective bulk flow of ISF, facilitated by the astrocytic aquaporin 4 (AQP4) water channels. The term “glymphatic system” was coined by Nedergaard, et al. in recognition of its dependence upon glial cells and the similarity of its functions to those of peripheral lymphatic system. Articles by Louveau et al. and Aspelund et al. reported that, the dural sinuses and meningeal arteries are lined with conventional lymphatic vessels, and that this long-elusive vasculature forms a connecting pathway to the glymphatic system.
With an observational point of view some relevant (in our opinion) scientific literature are analyzed to produce a global conclusion useful to the scope of this work. The literature observed or is found on PUBMED or in other OPEN LITERATURE. The keywords used are: Brain glymphatic system, Brain washing system, Vascular dementia, PD, DA, Diabetic dementia, New therapeutic strategy, Toxicology approach, Clearance of wasting molecule, Kinetics, Saturation, Genetic, Depurative approach. The period observed is included on the data of publications reported (recent and last year).
According scientific literature
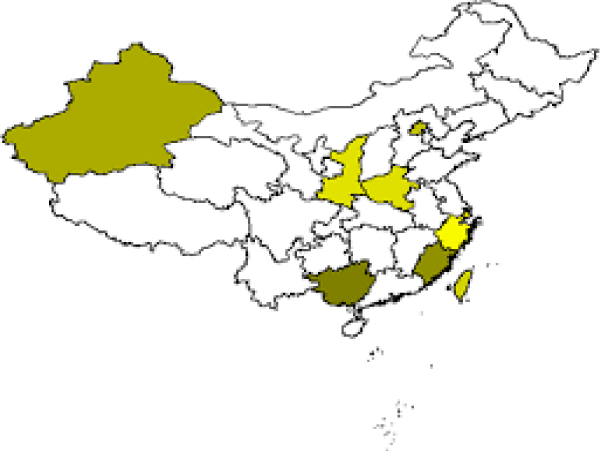
Wenyan Zou, et al. [1]: Abnormal aggregation of brain α-synuclein is a central step in the pathogenesis of Parkinson’s disease thus, it is reliable to promote the clearance of α-synuclein to prevent and treat PD (Figure 1). Recent studies have revealed an essential role of glymphatic system and meningeal lymphatic vessels in the clearance of brain macromolecules, however, their pathophysiological aspects remain elusive.
Figure 1: Parkinson’s disease in China.
Method: Meningeal lymphatic drainage of 18-week-old A53T mice was blocked via ligating the deep cervical lymph nodes. Six weeks later, glymphatic functions and PD-like phenotypes were systemically analyzed.
Results: Glymphatic influx of cerebrospinal fluid tracer was reduced in A53T mice, accompanied with peri-vascular aggregation of α-synuclein and impaired polarization of aquaporin 4 expression in substantia nigra. Cervical lymphatic ligation aggravated glymphatic dysfunction of A53T mice, causing more severe accumulation of α-synuclein, glial activation, inflammation, dopaminergic neuronal loss and motor deficits.
Conclusion: The results suggest that brain lymphatic clearance dys-function may be an aggravating factor in PD pathology.
PD pathology
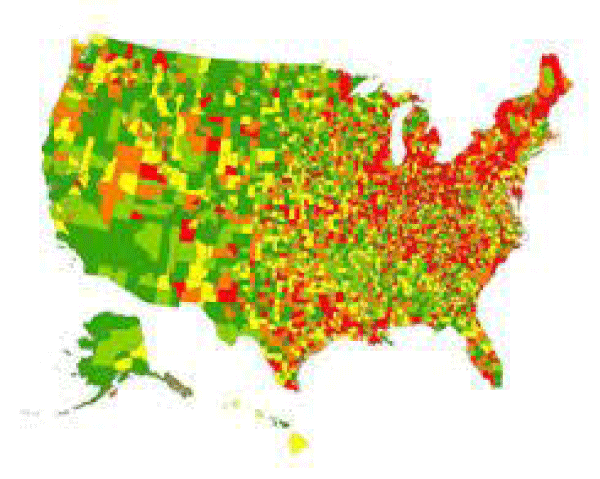
PD is an age-dependent neurodegenerative disease, characterized by progressive loss of midbrain dopaminergic neurons and formation of Lewy bodies. Although the pathogenesis of PD has not been completely elucidated, excessive accumulation of toxic forms of α-synuclein (α-syn) with a series of secondary pathological cascades plays crucial roles in the onset of PD. This is mainly due to an imbalance between production and clearance of α-syn in the brain. A small part of patients with early-onset PD show increased α-syn production because of mutations in the α-syn gene, while the vast majority of PD patients suffer from decreased removal of α-syn from the brain. Previous studies have reported that α-syn is degraded through various intracellular clearance mechanisms including auto-phagy lysosomal pathways. It is unclear whether there is an extracellular route to remove soluble α-syn directly from the brain. Exploring this issue will help to discover potentially new strategies for the prevention and treatment of PD. Recent findings have suggested that brain lymphatic drainage system including a brain-wide network of Para-vascular channels, termed the ‘glymphatic system’ and meningeal lymphatic vessels drain macromolecules from the brain parenchyma to the deep cervical lymph nodes (Dclns). Further evidence suggests that glymphatic function depends on astroglial aquaporin-4 (AQP4), contributing to a major portion of brain soluble amyloid-β (Aβ) clearance (Figure 2).
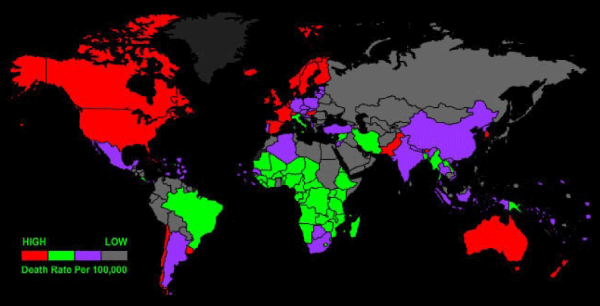
Figure 2: Parkinson’s disease in USA, the largest U.S. study of the epidemiology of Parkinson’s disease shows the highest prevelance (13,800 cases or more per 100,000 residents ages 65 and older) in red. Lower prevalence rates are progressively indicated by orange, yellow, light green and green.
Impaired glymphatic clearance exacerbates Aβ deposition in the brain of a mouse model of Alzheimer’s disease (AD). The glymphatic system facilitates convective exchange of various interstitial solutes including glucose, lipids, amino acids, lactic acid and neurotransmitters between cerebrospinal fluid (CSF) and interstitial fluid (ISF). Disruptionof meningeal lymphatic drainage aggravates parenchymal Aβ accumulation and cognitive decline of AD transgenic mice. It remains un-clear whether glymphatic clearance dysfunction is involved in brain α-syn aggregation and related pathology. To address this question, we investigated glymphatic clearance function of A53T mice over-expressing mutated human α-syn. The consequence of blocking meningeal lymphatic drainage on α-syn-related pathophysiology was also observed in the PD animal model. The finding suggests that impaired brain lymphatic clearance is involved in PD pathology. We have showed that blocking extracranial lymphatic drainage markedly exacerbates accumulation of α-syn in the brain parenchyma of A53T mice and subsequently leads to extensive reactive astrogliosis and AQP4 polarity destruction, thus exacerbating glymphatic clearance deficits and promoting α-syn-related pathology. It should be noted that the lymphatic system is rich in collateral circulation, likely after LDclns, compensatory effects of other extra-cranial lymphatic systems including the cribriform plate route need to be analyzed. The results suggest that both intracellular degradation and extra-cellular clearance of α-syn are impaired in A53T mice, but which one is an initial event needs to be further explored. Pathogenic point mutations in the α-syn gene, such as A53T and A30P missense mutations, are linked to familial PD. However, most neurodegenerative disorders involving Lewy bodies are associated with abnormal accumulation of wild-type α-syn. Neuronal over-expression of wild-type human α-syn in mice resulted in progressive accumulation of α-syn in neurons, associated with loss of dopaminergic terminals in the basal ganglia and with motor impairment. Whether brain lymphatic clearance dysfunction is also involved in pathology of this PD model and patients with AD warrants further investigation. Our results demonstrate an impairment of glymphatic clearance of α-syn in the brain of A53T mice, and disruption of meningeal lymphatic drainage further exacerbates α-syn-related pathology. Findings suggest that lymphatic clearance dysfunction is crucial in the onset and development of PD, and might be therapeutically targeted to alleviate age-associated neurodegenerative disorders.
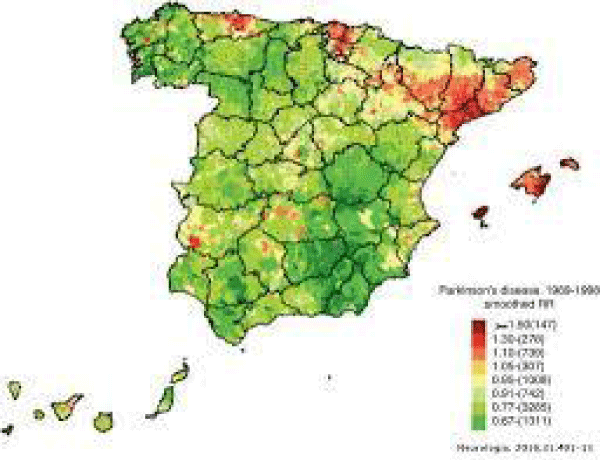
Our results demonstrate an impairment of glymphatic clearance of α-syn in the brain of A53T mice, and disruption of meningeal lymphatic drainage further exacerbates α-syn-related pathology. Findings suggest that lymphatic clearance dysfunction is crucial in the onset and development of PD, and might be therapeutically targeted to alleviate age-associated neurodegenerative disorders. According article “Brain Washing System-The System by Which the Wasting Molecules are Removed from Brain“ 2019, “The Central Nervous System (CNS) is the only organ system of the body which lacks its own waste clearance or lymphatic system, a system which helps in removal of metabolic byproducts and waste solutes. Although the brain plays its role in 25% metabolism of body and comprises only 2% of the total body mass, this high metabolic load needs a proficient system for the removal of waste solutes and for maintaining homeostasis of brain environment. Well-depicted components of waste removal comprise of perivascular fluid flow and phagocytic immune cell functions, nonetheless, the requirement for dynamic clearance of waste from the brain is getting progressively valued. Latest improvements in lymphatic vascular biology confront the recommendation that the brain deficits lymphatic removal system or an-equivalent. In this review article, a recently discovered waste removal system, the glymphatic system and its functioning is discussed, keeping in view the experimental studies performed on rodents. The glymphatic system is perivascular network dependent of glial cells that serves as a pseudo-lymphatic system in the brain. In the pathway of glymphatic system, cerebro-spinal fluid gains entry into brain by means of peri-arterial spaces, moves into the interstitial spaces through perivascular astrocytes and aquaporin-4 channels, and afterwards pushes the peri-venous waste of interstitial fluid (ISF) and its solutes into lymphatic vessels which eventually moves into systemic circulation. This system plays significant role individually and also in combination with authentic lymphatic system in drainage and getting clearance of wastes from the brain” (Figure 3).
Figure 3: Parkinson’s disease impact in Spain.
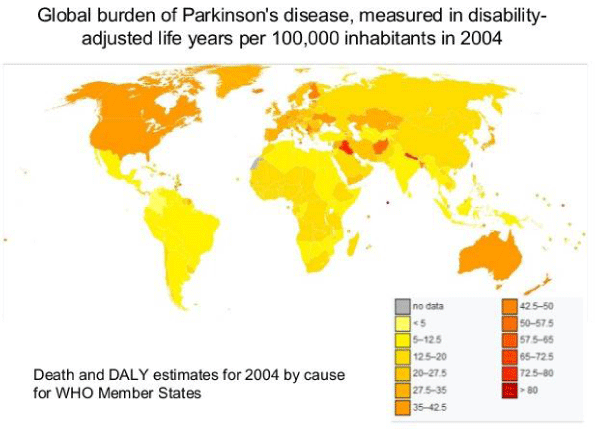
Abdelrahman Y. Fouda, et al. [2]: “The brain is a unique organ with no energy reserve. It requires constant blood flow to perform all the complex functions ranging from cognition to regulation of cardio-vascular homeostasis. Cerebral blood flow (CBF) is tightly regulated to meet the demands of the brain by providing constant flow at the right pressure, as well as delivering it to where it is needed the most as determined by brain activity. The cerebrovascular network comprised of arteries, arterioles, capillaries, venules, and veins plays a central role in achieving this high level of regulation as recently reviewed in the context of physiology and neuro-degenerative disorders. Its importance in cognitive function is increasingly recognized as discussed in a recent scientific statement on brain health from the American Heart Association. Reductions in CBF are known to occur in both vascular contributions to cognitive impairment/dementia (VCID) and Alzheimer disease (AD), but the role this phenomenon plays in the initiation and progression of the dementias is not as clear. Whereas hypoxia/ischemia is thought to be the initiating event in VCID, many believe that blood flow changes in AD occur in response to the neurodegeneration. It remains a strong possibility, however, that vascular dysfunction is a key precipitating event in both conditions given that they share the common vascular risk factors of hypertension, diabetes mellitus, and aging, among others (Figures 4,5).
Figure 4: Global burden of Parkinson’s disease, measured in disability adjusted life years per 100,000 inhabitants in 2004.
Figure 5: Prevalence of Dementia/Alzheimer’s globally.
There is a complex interaction between the brain and the cerebral vasculature to meet the metabolic demands of the brain for proper function. Preservation of cerebro-vascular function and integrity has a central role in this sophisticated communication within the brain, and any derangements can have deleterious acute and chronic consequences. In almost all forms of cognitive impairment, from mild to Alzheimer disease, there are changes in cerebrovascular function and structure leading to decreased cerebral blood flow, which may initiate or worsen cognitive impairment. In this focused review, we discuss the contribution of 2 major vasoactive pathways to cerebrovascular dysfunction and cognitive impairment in an effort to identify early intervention strategies.
Visual overview-an online visual overview
According to “Endogenus Toxicology: Modern Physio- Pathological Aspects and Relationship with New Therapeutic Strategies. An Integrative Discipline Incorporating Concepts from Different Research Discipline like Biochemistry, Pharmacology and Toxicology” 2019 and “Many pathologic diseases can be considered as related to an Endogenous toxicological moves and in time dependent way. In this work starting from the analysis of relevant literature involved with different disease and related to the endogenous local micro- environment some global conclusion useful as new tools for innovative pharmacological strategies will be submitted to the researcher. Physiology, pathology concept linked to the endogenous toxicological local micro-environment as new research instruments. Many pathological processes are strictly linked to endogenous local environmental conditions time related. Iper-bilirubinemia in newborn and ASD, Glicosilation in diabetes, lipids in Atersoclerotic process, Neuronal cell inclusion in Amyotrofic lateral sclerosis and Dementia, the Metastatic process, Oxidative stress in some cancer in example due by Cr (VI) and many other examples show an undeniable role of Exogenous but also by endogenous factors (Figures 6,7). A new scientific discipline named endogenous toxicology must be introduced as a useful instrument to better clear some pathological process and also to introduce better and new pharmacological (and not) strategies. What is relevant is to consider some pathology under a toxicological aspect and endogenous process time related. (Topographic condition, time of exposure, catabolic status). A better knowledge related basic pathologic process make possible to verify and introduce new therapeutic strategies to achieve better global clinical results. Concepts from toxicological sciences like dosage, time of exposure, kinetics, metabolism, dynamics and other must be applied also in endogenous toxicological-pathological process.
Figure 6: Neurodegeneration hypothesys, a vicious cycle of pathological neurovascular changes in the brain leads to a malignantly progressive burden of neurodegeneration.
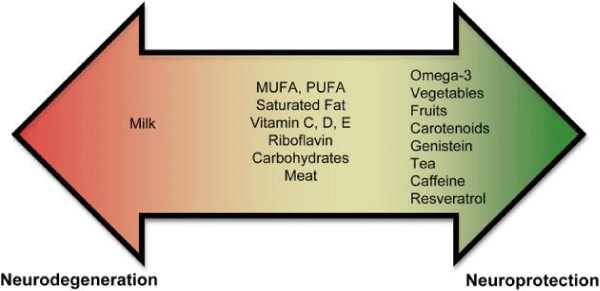
Figure 7: Diet component and effect.
Like classic toxicology science concept like:
• Endogenous- local Toxic substantives
• Topography of the toxic- process
• Metabolism-catabolism of this toxics
• Measure methods
• Kinetics, dynamics
• Dose- response relationship
• Risk factors
• Worsening local endogenous conditions, Additive condition
• Preventing strategies
• Depurative methods, inactivating- methods
• Antidotes approach
Ranjini K. Sundaram, et al. [3]: “The present study shows that treatment with detox gel in AD mice can be effective as a therapeutic method for lowering Aβ levels in the APPSWE mice (Tg2546). The high affinity sequestering ability combined with the lack of immunogenicity and capacity to improve memory parameters make it an interesting drug candidate for the treatment of AD. Thus our detox gel therapy might become a welcome non-immune based therapy with a future in human clinical settings” and “Quantitative analysis of Aβ-42 in brain Aβ in brain was extracted by methods described in experimental methods section. Aβ-42 levels were measured in brain extracts by an ELISA using a commercial antibody. The 2 step extraction method that we employed yields total Aβ. The results show that there is a reduction in the level of total Aβ-42 by 30% in the group of mice that received the detox gel when compared to the untreated group with a statistical significance (p < 0.001)” novel Detox Gel Depot sequesters β-Amyloid Peptides in a mouse model of AD”.
Dong SK, et al. [4]: “Molecular communications in the gut–brain axis, between the central nervous system and the gastrointestinal tract, are critical for maintaining healthy brain function, particularly in aging. Epidemiological analyses indicate type 2 diabetes mellitus (T2DM) is a risk factor for neurodegenerative disorders including Alzheimer’s disease and Parkinson’s diseases for which aging shows a major correlative association. Common patho-physiological features exist between T2DM, AD, and PD, including oxidative stress, inflammation, insulin resistance, abnormal protein processing, and cognitive decline, and suggest that effective drugs for T2DM that positively impact the gut–brain axis could provide an effective treatment option for neurodegenerative diseases. Glucagon-like peptide-1 (GLP-1)-based anti-diabetic drugs have drawn particular attention as an effectual new strategy to not only regulate blood glucose but also decrease body weight by reducing appetite, which implies that GLP-1 could affect the gut–brain axis in normal and pathological conditions. The neurotrophic and neuro-protective effects of GLP-1 receptor (R) stimulation have been characterized in numerous in vitro and in vivo preclinical studies using GLP-1R agonists and dipeptidyl peptidase-4 inhibitors. The first open label clinical study of exenatide, a long-acting GLP-1 agonist, in the treatment of PD showed long-lasting improvements in motor and cognitive function. Herein, we review the physiological role of the GLP-1R pathway in the gut–brain axis and the therapeutic strategy of GLP-1R stimulation for the treatment of neurodegenerative diseases focused on PD, for which age is the major risk factor”.
Ling Wang, et al. [5]: “Parkinson’s disease is a common disorder, and the diagnosis of Parkinson’s disease is clinical and relies on the presence of characteristic motor symptoms. The accuracy of the clinical diagnosis of PD is still limited. Functional neuroimaging using SPECT technique is helpful in patients with first signs of Parkinsonism. The changes detected may reflect the disease process itself and/or compensatory responses to the disease, or they may arise in association with disease- and/or treatment-related complications. Age is the largest risk factor for the development and progression of PD. PD may reflect a failure of the normal cellular compensatory mechanisms in vulnerable brain regions, and this vulnerability is increased by ageing. PD is one of the best examples of an age-related disease. Pres-ynaptic imaging has demonstrated the ability to objectively measure the progression of Parkinson’s disease. Although the rate of progression of the dopaminergic terminal loss in patients with PD was correlated with clinical severity, the annual percentage loss of 123I-β-CIT striatal uptake did not correlate with the annual loss in measures of clinical function. Striatal 123I-IPT uptake was closely related to the stage of PD. The binding ratios decreased markedly from H-Y stage I to stage IV; this imaging technique has a special advantage that data can be acquired within a few hours after injection. The rate of progression may be faster in APS than in PD. To interpret the results well, caution must be paid in the studies in which therapeutic effects in Parkinson’s disease were also monitored by serial imaging of nigro-striatal neurons”.
Jiang W, et al. [6]: “Dairy foods have been linked to Parkinson’s disease, and a meta-analysis of prospective cohort studies on dairy foods intake and PD risk was conducted. Eligible studies were identified in a literature search of EMBASE and PubMed up to April 2014. Seven results from prospective studies were included, including 1,083 PD cases among 304,193 subjects. The combined risk of PD for highest vs. lowest level of dairy foods intake was 1.40 (1.20 - 1.63) overall, 1.66 (1.29 - 2.14) for men and 1.15 (0.85 - 1.56) for women. For highest vs. lowest level, the PD risk was 1.45 (1.23 - 1.73) for milk, 1.26 (0.99 - 1.60) for cheese, 0.95 (0.76 - 1.20) for yogurt and 0.76 (0.51 - 1.13) for butter. The linear dose-response relationship showed that PD risk increased by 17% [1.17 (1.06 - 1.30)] for every 200 g/day increment in milk intake (Pfor non-linearity = 0.22), and 13% [1.13 (0.91 - 1.40)] for every 10 g/day increment in cheese intake (Pfor non-linearity = 0.39). The absolute risk differences were estimated to be 2 - 4 PD cases per 100,000 person-years for every 200 g/day increment in milk intake, and 1 - 3 PD cases per 100,000 person-years for every 10 g/day increment in cheese intake. Dairy foods (milk, cheese) might be positively associated with increased risk of PD, especially for men“.
Stacey E. Seidl, et al. [7]: “PD is the second most prevalent neurodegenerative disease in ageing individuals. It is now clear that genetic susceptibility and environmental factors play a role in disease etiology and progression. Because environmental factors are involved with the majority of the cases of PD, it is important to understand the role nutrition plays in both neuro-protection and neuro-degeneration. Recent epidemiological studies have revealed the promise of some nutrients in reducing the risk of PD. In contrast, other nutrients may be involved with the etiology of neuro-degeneration or exacerbate disease progression. This review summarizes the studies that have addressed these issues and describes in detail the nutrients and their putative mechanisms of action in PD. Nutrients that may be associated with an increased risk or progression of PD. Dairy product consumption and drinking milk may increase one’s risk of PD independently of calcium intake particularly in men.
Nonetheless, a positive association between milk consumption and PD risk was also observed in women in one study. Preliminary research shows that individuals who consume large amounts of dairy products may often have low serum uric acid levels. Serum uprate and uric acid is inversely correlated with the risk of PD and disease duration. The neuro-protective effects of serum uprate may be limited to men since the same is not observed in women. In addition, the possible presence of dopaminergic neurotoxins, including pesticides and poly-chlorinated biphenyls in dairy products may increase the risk of PD. Accordingly, postmortem studies show higher levels of organo-chlorines, including dieldrin, an organo-chlorine pesticide, and polychlorinated biphenyls in the brains of PD patients compared to non-neurological controls. Yet, the presence of dopaminergic neurotoxins may not be the only component responsible for the relationship between dairy products and PD. In fact, a strong positive association with the consumption of milk, but not cheese or yoghurt has been reported. Other constituents in milk may be detrimental with regards to PD and additional studies are needed in order to identify them. The association between dairy products and PD should be interpreted with caution, however, as other studies have found conflicting results.
“Currently, there is an abundance of preliminary evidence that indicates that some nutrients may increase an individual’s risk for PD, while others may be neuroprotective. These results are not unexpected since nutrients affect mitochondrial energy function and provide vital antioxidant functions that ameliorate the free-radical byproducts of oxidative phosphorylation. A poor diet may lead to increased oxidative stress, which could impede the antioxidant defense system. In contrast, a well-balanced diet rich in a variety of foods, including numerous servings of vegetables and fruits (especially those containing nicotine) and moderate amounts of omega-3 fatty acids, tea, caffeine, and wine may provide neuro-protection. In spite of promising effectiveness of these nutrients in PD, we lack definitive evidence-based answers as a result of limited large prospective randomized controlled studies designed to address these issues. Indeed, there are several limitations in some epidemiological studies assessing dietary factors and PD that merit further attention. The assumption that dietary patterns remain unchanged over time is a major limitation. Information on diet during development would be very helpful and may weaken or strength a result. Patients with PD may experience non-motor symptoms at early stages such as constipation, dysphagia, depression, and hyposmia that may affect dietary choices and therefore may be responsible for the impairment of nutritional status observed in PD. These factors may remain undetected and therefore not properly reported. Incorporation of these critical factors into clinical practice and epidemiological studies will greatly improve the reliability of studies assessing the role of nutrients in PD”.
Anderson C, et al. [8]: The association between self-reported past food intake and PD was investigated in a case-control study of men and women aged 40-89 years. Newly- diagnosed idiopathic PD cases were ascertained from neurologists, and from outpatient and pharmacy computerized -databases, at the Group Health Cooperative (GHC) clinics in the Puget Sound region of Washington state. Control subjects were chosen from the GHC patient roster and had no reported history of diagnosed neurodegenerative disease. Dietary data were obtained from structured questionnaires. An increase in PD risk with increasing intake was noted for foods that contain animal fat and foods containing vitamin D. Intake of fruits, vegetables, meats, bread and cereals, or foods containing vitamins A, C, E, or iron was not significantly related to PD risk. Vitamin use, in general, was also not found to be related to PD risk, although a significant trend of increasing risk of PD was noted for intake of vitamin A- supplements. Although these data support previous- findings of no association of past intake with most food groups and PD risk, they confirm an increased risk of PD associated with foods containing animal fat“.
Geir Ringstad, et al: “Knowledge about the access of substances administered in the subarachnoid space to human brain as whole could potentially make new treatments of brain disease. The blood-brain barrier (BBB) represents one of the largest obstacles to effective CNS drug delivery (1), and the compartment within blood vessels merely occupies less than 3% of the total brain volume (2). Thus, new therapeutic CNS drugs generally show lower success rates than those for non-CNS indications (3), while intrathecal treatment regimens have emerged with great promise. Previous animal studies have shown communication between the subarachnoid cerebrospinal fluid (CSF) space and perivascular compartments of the brain and spinal cord. Literature reporting human in vivo CSF tracer studies is lacking, and observations made in animals have not been translated into humans. Two recent human studies demonstrated brain parenchyma enhancement subsequent to subarachnoid (intrathecal) administration of a MRI contrast agent, but observations were limited to select ROIs. An MRI study of rats failed to demonstrate enhancement in deep brain white matter. Animal studies report diverging findings regarding the sites of perivascular brain influx and efflux and direction of perivascular flow. Animal in vivo observations typically cover extremely limited fields of view, as when utilizing 2-photon microscopy. Mechanisms behind transport and clearance of substances within the brain interstitial space are controversial. For a long time, size-dependent diffusion was considered to explain interstitial movement of molecules.
In 2012, a brain-wide pathway for convective transport of waste solutes from the brain was first described and denoted the glymphatic system. Net water and solute transport through the extracellular compartment from arterial to venous paravascular spaces was proposed to be dependent on aquaporin-4 (AQP4) water channels and mediated by arterial pulsations. Impaired glymphatic function has been suggested to be instrumental in a range of brain diseases; this has been illustrated most in Alzheimer’s dementia but has also been shown to be relevant in posttraumatic encephalopathy, ageing, sleep, depression, and exercise. The glymphatic concept has been challenged by several modeling studies that have opposed CSF pulsations as the explanation for net convective interstitial flow. A later animal study utilized a similar set of experiments as those bringing evidence for a glymphatic system and found, contrary to the previous observations, that interstitial flow could be explained by diffusion alone, independent of AQP4 status. Later, several independent groups provided evidence for an important role of AQP4 in glymphatic circulation. To this end, we here show for what we believe to be the first time brain-wide CSF tracer enhancement and clearance in humans. For this, we administered an MRI contrast agent in the sub-arachnoid CSF compartment, followed by repeated MRI scans at 24 and 48 hours and after 4 weeks. In addition, we found delayed clearance of CSF tracer from the brain in a cohort of patients with dementia and expected CSF circulation failure (idiopathic normal pressure hydrocephalus [iNPH])”.
Iliff JJ, et al. [9]: Because it lacks a lymphatic circulation, the brain must clear extracellular proteins by an alternative mechanism. The cerebrospinal fluid (CSF) functions as a sink for brain extracellular solutes, but it is not clear how solutes from the brain- interstitium move from the parenchyma to the CSF. We demonstrate that a substantial portion of subarachnoid CSF cycles through the brain interstitial space. On the basis of in vivo two-photon imaging of small fluorescent tracers, we showed that CSF enters the parenchyma along para-vascular spaces that surround penetrating arteries and that brain interstitial fluid is cleared along para-venous drainage pathways. Animals lacking the water channel aquaporin-4 (AQP4) in astrocytes exhibit slowed CSF influx through this system and a ~70% reduction in interstitial solute clearance, suggesting that the bulk fluid flow between these anatomical influx and efflux routes is supported by astrocytic water transport. Fluorescent-tagged amyloid β, a peptide thought to be pathogenic in Alzheimer’s disease, was transported along this route, and deletion of the Aqp4 gene suppressed the clearance of soluble amyloid β, suggesting that this pathway may remove amyloid β from the central nervous system. Clearance through para-venous flow may also regulate extracellular levels of proteins involved with neurodegenerative conditions, its impairment perhaps contributing to the mis-accumulation of soluble proteins“.
Hedok Lee, et al. [10]: “The glymphatic pathway expedites clearance of waste, including soluble amyloid β (Aβ) from the brain. Transport through this pathway is controlled by the brain’s arousal level because, during sleep or anesthesia, the brain’s interstitial space volume expands (compared with wakefulness), resulting in faster waste removal. Humans, as well as animals, exhibit different body postures during sleep, which may also affect waste removal. Not only the level of consciousness, but also body posture, might affect CSF–interstitial fluid (ISF) exchange efficiency. We used dynamic-contrast-enhanced MRI and kinetic modeling to quantify CSF-ISF exchange rates in anesthetized rodents’ brains in supine, prone, or lateral positions. To validate the MRI data and to assess specifically the influence of body posture on clearance of Aβ, we used fluorescence microscopy and radioactive tracers, respectively. The analysis showed that glymphatic transport was most efficient in the lateral position compared with the supine or prone positions. In the prone position, in which the rat’s head was in the most upright- position (mimicking posture during the awake state), transport was characterized by “retention” of the tracer, slower clearance, and more CSF efflux along larger caliber cervical vessels. The optical imaging and radiotracer studies confirmed that glymphatic transport and Aβ clearance were superior in the lateral and supine positions. We propose that the most popular sleep posture (lateral) has evolved to optimize waste removal during sleep and that posture must be considered in diagnostic imaging procedures developed in the future to assess CSF-ISF transport in humans. The rodent brain removes waste better during sleep or anesthesia compared with the awake state.
Animals exhibit different body posture during the awake and sleep states, which might affect the brain’s waste removal efficiency. We investigated the influence of body posture on brain wide transport of inert tracers of anesthetized- rodents. The major finding of our study was that waste, including Aβ, removal was most efficient in the lateral position (compared with the prone position), which mimics the natural resting/sleeping position of rodents. Although our finding awaits testing in humans, we speculate that the lateral position during sleep has advantage with regard to the removal of waste products including Aβ, because clinical- studies have shown that sleep drives Aβ clearance from the brain.
Maiken Nedergaard, et al. [11]: “Essentially all neuro-degenerative diseases are associated with mis-accumulation of cellular waste products. Of these, misfolded or hyperphosphorylated proteins are among the most difficult for the brain to dispose. Tau and β-amyloid can accumulate as stable aggregates that are neurotoxic in conditions such as AD. Intra-cellular proteasomal degradation and autophagy are considered the principal means for removing proteins in the CNS, and the dysfunction of each has been causally associated with neuro-degeneration. Yet many cytosolic proteins are released into the interstitial space in the brain, suggesting that extracellular disposal routes may also eliminate waste. Throughout the body’s tissues, bulk flow of the fluid between cells, into the blood or lymph, plays an important role in the removal of potentially toxic metabolic by-products. Lymphatic vessels, which run in parallel with the blood vascular system, are the principal means by which tissues eliminate excess fluid and proteins. Although the density of lymph vessels generally correlates with tissue metabolic rate, the brain and spinal cord are curiously devoid of such a lymphatic tree. This is puzzling because the high metabolic activity of neurons predicts the need for rapid elimination of their metabolic- byproducts. It was long thought that movement of the cerebrospinal fluid (CSF), which is produced in the choroid plexus of the brain and flows through its ventricles and basal cisterns, constitutes a “sink” for waste products to diffuse from the brain, for eventual clearance to the general-circulation. The large tissue distances in most of the brain prevent diffusion and bulk flow from making this process efficient.
Albumin, for instance, would require more than 100 hours to diffuse through 1 cm of brain tissue. 2-photon imaging of live mice through a closed cranial window has since permitted the direct observation of CSF movement through the intact brain. This technique revealed that CSF is exchanged rapidly with interstitial fluid in the brain by a highly organized, brain-wide pathway that consists of three serial elements: a para-arterial CSF influx route, a para-venous ISF clearance route, and an intracellular Tran’s astrocytic path that couples the two extra-cellular para-vascular routes. CSF passes through the para-arterial space that surrounds arteries; the space is bound by the ab-luminal surface of the blood vessel and the apical processes of astrocytes. Water channels called aquaporin 4 (AQP4) on the vascular end feet of astrocytes facilitate convective flow out of the para-arterial space and into the interstitial space. As CSF exchanges with the ISF, vectorial convective fluxes drive waste products away from the arteries and toward the veins. ISF and its constituents then enter the para-venous space. As ISF exits the brain through the para-venous route, it reaches lymphatic vessels in the neck, and eventually returns its contents to the systemic circulation. Radio-label tracer studies indicate that 40% to 80% of NIH-PA large proteins and solutes are removed from the brain through this macroscopic clearance pathway. CSF can also exit through the arachnoid villi, which extend through the outer protective membrane layer of the brain and allow CSF to exit to the blood-stream, as well as at sites along the cavity and cranio-spinal nerve roots. Regardless of the route, its solutes and proteins ultimately reach the liver, where they are degraded.
As such, the “glymphatic system” so called for its dependence on glial water channels and its adoption of a clearance function similar to that of the peripheral lymphatic system avoids the need for local protein processing and degradation. Instead, it facilitates transport to the same central excretion and recycling sites used by other tissues. Studies of mice genetically engineered to lack AQP4 showed that fluid flux through the glymphatic pathway relies on specific expression of this water channel along the apical membrane of vascular end-feet of astrocytes. When AQP4 is mis-located to the cell body of astrocytes or to astrocytic- processes that do not about the vasculature, as observed in traumatic brain injury or stroke, clearance of soluble proteins through the glymphatic system declines substantially. The injection of brain extracts from mice containing an aggregation-prone form of human tau protein, into the brains of mice expressing wild-type human tau, induces self-assembly of the wild-type human tau into filaments. This results in the pathological spread of tau aggregates from the injection site to distant brain regions. Perhaps the most persuasive example of CSF recycling as the cause of dispersing the initial seeds of tau tangles is after traumatic brain injury. As a result of axon damage, the tau concentration in CSF increases by as much as a factor of 40,000. Consequently, as the heavily tau-laden CSF enters the brain tissue through the para-arterial space, it is taken up by cells closest to the para-vascular boundary, thereby generating the typical para-vascular predominance of tau-immuno-reactive neurofibrillary tangles, glymphatic CSF influx may also act as a constant source for delivering β-amyloid, which could contribute to the growth of para-arterial deposits in cerebral amyloid angiopathy. In turn, the same para-arterial space that normally functions as a low-resistance influx path for CSF will narrow as the amyloid plaques enlarge, slowing glymphatic clearance and thus accelerating amyloid deposition. As such, studies of the multiple pathways involved in glymphatic clearance may identify new targets for treating neurodegenerative diseases. mislocation of AQP4 water channels may contribute to neurodegenerative disease progression. Thus, potentiating the insertion and activity of AQP4 channels in astrocytic vascular end-feet might mitigate or even reverse the course of protein-associated neurodegenerative disorders. Can the efficiency of glymphatic clearance be assessed?
Preclinical analysis in rats shows that magnetic resonance imaging can provide a brain-wide map of both glymphatic influx and efflux, by which clearance kinetics can be derived and compared across subjects. By extending this approach to humans, it may be possible to identify patients at risk for Developing AD who would benefit from therapeutic intervention before symptomatic neuro-degeneration ensues. Similarly, this type of analysis might allow the monitoring of treatment responses, as well as the identification of genetic markers that predict enhanced susceptibility to glymphatic decline. Recognition that the brain, like all other organs, uses both local and organ-wide mechanisms for clearing interstitial protein waste may offer new insights into the patho-physiology and prophylaxis of neuro-degeneration, as well as injuries and protein-pathies of the human CNS”.
Leonidas Stefanis, et al: “A-Synuclein is a presynaptic neuronal protein that is linked genetically and neuropathologically to Parkinson’s disease (PD). a-Synuclein may contribute to PD pathogenesis in a number of ways, but it is generally thought that its aberrant soluble oligomeric conformations, termed proto-fibrils, are the toxic species that mediate disruption of cellular homeostasis and neuronal death, through effects on various intracellular targets, including synaptic function. secreted a-synuclein may exert deleterious effects on neighboring cells, including seeding of aggregation, thus possibly contributing to disease propagation. Although the extent to which a-synuclein is involved in all cases of PD is not clear, targeting the toxic functions conferred by this protein when it is dys-regulated may lead to novel therapeutic strategies not only in PD, but also in other neuro-degenerative conditions, termed synucleino-pathies”.
Lee HJ, et al. [12]: “Cytoplasmic deposition of alpha-synuclein aggregates is a common pathological feature of many neurodegenerative diseases. Strong evidence for the causative role of alpha-synuclein in these disorders is provided by genetic linkage between this gene and familial Parkinson’s disease and by neurodegeneration in transgenic animals that overexpress this protein. In particular, it has been hypothesized that the accumulation of non-fibrillar oligomers of alpha-synuclein, which serve as intermediates for fibrillar inclusion body formation, causes neuro-degeneration. Little is known about how cells handle potentially toxic protein aggregates. Here we demonstrate that cells are capable of clearing preformed alpha-synuclein aggregates via the lysosomal degradation pathway. Consequently, blocking this pathway causes the accumulation of the aggregates in non-neuronal cells, differentiated neuro-blastoma cells, and primary cortical neurons. This aggregate clearance occurs in an aggregation stage-specific manner; oligomeric intermediates are susceptible to clearance, whereas mature fibrillar inclusion bodies are not. Neutralization of the acidic compartments leads to the accumulation of alpha-synuclein aggregates and exacerbates alpha-synuclein toxicity in post-mitotic neuronal cells, suggesting that the accumulation of oligomeric intermediates may be an important event leading to alpha-synuclein-mediated cell death. These results suggest that enhancing lysosomal function may be a potential therapeutic strategy to halt or even prevent the pathogenesis of PD and other Lewy body diseases”.
O Marques, T F Outeiro, et al. [13]: “Alpha-synuclein is associated with both familial and idiopathic cases of PD. The precise function of alpha-synuclein remains equivocal. Alpha-synuclein misfolds and forms protein aggregates in Parkinson’s disease and in various in vitro and in vivo models of synucleino-pathies. Alpha-synuclein has been detected in human and mouse CSF and in the media of cultured cells”.
Bao-Liang Suna, et al. [14]: “The belief that the vertebrate brain functions normally without classical lymphatic drainage vessels has been held for many decades. On the contrary, new findings show that functional lymphatic drainage does exist in the brain. The brain lymphatic drainage system is composed of basement membrane-based perivascular pathway, a brain-wide glymphatic pathway, and cerebrospinal fluid drainage routes including sinus-associated meningeal lymphatic vessels and olfactory/cervical lymphatic routes. The brain lymphatic systems function physiological as a route of drainage for interstitial fluid from brain parenchyma to nearby lymph nodes. Brain lymphatic drainage helps maintain water and ion balance of the ISF, waste clearance, and reabsorption of macromolecular solutes. A second physiological function includes communication with the immune system modulating immune surveillance and responses of the brain. These physiological functions are influenced by aging, genetic phenotypes, sleep-wake cycle, and body posture. The impairment and dysfunction of the brain lymphatic system has crucial roles in age related changes of brain function and the pathogenesis of neuro-vascular, we summarize the key component elements (regions, cells, and water transporters) of the brain lymphatic system and their regulators as potential therapeutic targets in the treatment of neurologic diseases. We highlight the clinical importance of ependymal route-based targeted gene therapy and intra-nasal drug administration in the brain by taking advantage of the unique role played by brain lymphatic pathways in the regulation of CSF flow and ISF/CSF exchange. The brain lymphatic drainage system consists of the BM perivascular pathway, glymphatic system, the olfactory/cervical lymphatic drainage route and the meninges of a lymphatic network.
The driving forces moving fluids and solutes in these pathways rely on vessel pulsations, intracranial pressure, osmotic gradients and various transporters. Even though co-existence of both perivascular pathway and glymphatic circulation is still a subject of debate, the integrated function of brain lymphatic drainage system, through selective actions and complementary effects of each component, is essential for maintaining homeostasis of the brain. As a unique lymphatic route, the brain lymphatic drainage system may serve as a pathway to clean ISF with its constituent molecules and wastes from the brain as well as an independent highway for conveyance of lipid signaling molecules. The brain lymphatic drainage system participates in immune responses and surveillance. These networks are affected by aging, genetic factors, sleep-wake cycle, and body posture. The clearance efficiency is influenced by different pathological conditions or diseases that change the composition of the constituent proteins, decrease vascular plasticity, inactive aquaporin water channels or other transporters, and obstruct perivascular cannels.
The impairment of the brain lymphatic drainage system results in failure of solute clearance, leading to dys-homeostasis and accumulation of neuro-toxic solutes that are of clinical relevance to AD. On the other hand, AD immune-therapy may induce the formation of the immune complex at the BM of cerebral arteries, which further weakens perivascular clearance of soluble Ab and increases severity of CAA. The blood-brain barrier, as “a gatekeeper” between the CNS and other parts of the body, restricts macromolecules entering into the CNS. The features of the brain lymphatic drainage pathways provide a unique opportunity for intracranial drug delivery and gene therapies via the dynamics of CNS fluids and exchange of CSF/ISF. Applications of ependymal route-based gene therapy and intranasal drug delivery or immune-therapy in the brain are good examples. These targeting strategies of drug administration based on the brain lymphatic drainage system bypass the limitations of the blood brain barrier, maintain effective drug concentration in brain while avoiding or decreasing the peripheral side effects of brain targeted drugs. Novel tracers and minimally or non-invasive techniques have been used widely in studies on the brain lymphatic drainage pathways and related neurological diseases. The progress in new techniques has been mainly made in the following fields: 1) in vivo 2-photon imaging using different types of Fluorescent tracers. The choice of such tracers may be based on their molecular weight, size, lipophilic or hydrophilic characters, cell membrane permeability, and clinical relevance to in vivo imaging of signal molecules.
Natural solutes such as Ab can be directly used as tracers showing their retention and clearance route. 2) Lymphatic cell reporter mice and newly established dissection of mouse meninges; 3) single-molecule force spectros- copy. This AFM-based technique is able to measure specific interaction forces such as attachment or adhesion forces at the single-molecule level (for example, the force for Ab/ApoE4 binding to the BM constitutive proteins; 4) Cerebrospinal fluid dynamics; and 5) ultrafast magnetic resonance encephalography to image brain fluid dynamics. More interestingly, in 3D tissue engineering of CNS modeling, reconstitution of the brain functional lymphatic system will be a challenging issue. New bio-markers have been identified to monitor the changes in the brain lymphatic system in clinical studies, such as: 1) determination of enlarged PVS using high-resolution 3D MR imaging; 2) Ab scanning by using an amyloid plaque biomarker Pittsburgh compound B for detecting cerebro-vascular deposition of the Ab peptide; 3) the assessment of vascular pulsation. The arterial stiffness can be determined by automated measurement of pulse wave velocity in vascular beds; 4) in vivo measurement of perfusion of the glymphatic system by contrast-enhanced MRI with intra-thecal gadolinium contrast medium to study water and solute transport in the human brain and 5) real-time MRI measurements of ADC value, reflecting regional edema of brain.
CSF profiles or biomarkers such as Tau have been validated in neuro-degenerative and vascular dementias and higher CSF pH observed during the developing course of delayed cerebral ischemia after aneurysmal SAH. Progress in understanding the mechanisms functioning to regulate the brain lymphatic drainage system not only helps us comprehend CNS function, but also provides exciting new therapeutic targets for treatment of human neurological- diseases or disorders”.
Iliff JJ, et al. [15]: Traumatic brain injury (TBI) is an established risk factor for the early development of dementia, including AD and the post-traumatic brain frequently exhibits neurofibrillary tangles comprised of aggregates of the protein tau. We have recently defined a brain-wide network of para-vascular channels, termed the “glymphatic” pathway, along which CSF moves into and through the brain parenchyma, facilitating the clearance of interstitial solutes, including amyloid-β, from the brain. Here we demonstrate in mice that extracellular tau is cleared from the brain along these para-vascular pathways. After TBI, glymphatic pathway function was reduced by ∼60%, with this impairment persisting for at least 1 month post injury. Genetic knock-out of the gene encoding the astroglial water channel aquaporin-4, which is importantly involved in para-vascular interstitial solute clearance, exacerbated glymphatic pathway dysfunction after TBI and promoted the development of neurofibrillary pathology and neuro-degeneration in the post-traumatic brain. These findings suggest that chronic impairment of glymphatic pathway function after TBI may be a key factor that renders the post-traumatic brain vulnerable to tau aggregation and the onset of neuro-degeneration“.
Rasmussen MK, et al. [16]: The glymphatic (glial-lymphatic) pathway is a fluid-clearance pathway identified in the rodent brain in 2012. This pathway subserves the flow of CSF into the brain along arterial perivascular spaces and subsequently into the brain interstitium, facilitated by aquaporin 4 (AQP4) water channels. The pathway then directs flow towards the venous perivascular and per-neuronal spaces, ultimately clearing solutes from the neuropil into meningeal and cervical lymphatic drainage vessels. In rodents, the glymphatic pathway is predominantly active during sleep, when the clearance of harmful metabolites such as amyloid β (Aβ) increases two-fold relative to the waking- state. Glymphatic dysfunction, probably related to perturbed AQP4 expression, has been shown in animal models of traumatic brain injury, Alzheimer’s disease, and stroke. The recent characterizations of the glymphatic and meningeal lymphatic systems in rodents and in humans call for revaluation of the anatomical routes for CSF-interstitial fluid flow and the physiological role that these pathways play in CNS health. Several features of the glymphatic and meningeal lymphatic systems have been shown to be present in humans. MRI scans with intra-ethically administered contrast agent show that CSF flows along pathways that closely resemble the glymphatic system outlined in rodents. Furthermore, PET studies have revealed that Aβ accumulates in the healthy brain after a single night of sleep deprivation, suggesting that the human glymphatic pathway might also be primarily active during sleep. Other PET studies have shown that CSF clearance of Aβ and tau tracers is reduced in patients with Alzheimer’s disease compared with healthy controls.
The observed reduction in CSF clearance was associated with increasing grey-matter concentrations of Aβ in the human brain, consistent with findings in mice showing that decreased glymphatic function leads to Aβ accumulation. Altered AQP4 expression is also evident in brain tissue from patients with Alzheimer’s disease or normal pressure hydro-cephalus; glymphatic MRI scans of patients with normal pressure hydrocephalus show reduced CSF tracer entry and clearance. WHERE NEXT? Research is needed to confirm whether specific factors driving glymphatic flow in rodents also apply to humans. Longitudinal imaging studies evaluating human CSF dynamics will determine whether a causal link exists between reduced brain solute clearance and the development of neuro-degenerative diseases. Assessment of glymphatic function after stroke or traumatic brain injury could identify whether this function correlates with neurological recovery. New insights into how behaviour and genetics modify glymphatic function, and how this function decompensates in disease, should lead to the development of new preventive and diagnostic tools and novel therapeutic targets”.
Experimental project: In order to verify relationship between some environmental factors and neurodegenerative condition is useful to compare epidemiology of some of this disease to the diet habit or factors like Advance or not advanced countries status. A diet like in advanced countries can be related to a saturation of brain wasting system? At the same time observing the data related migration of people form south of the world to the north and incidence of neurodegenerative disease. Observing the distribution of this characteristic is possible to say that in world point of view this are a great volume of data and so a significative situation. All this under this facts: About 25% of calories more than in industrialized countries vs. not. In south of the world 61% of total calories come from cereals, Iper-proteic diet (meat) in north of the world.
Related the analysis of literature presented and to the experimental hypotesys presented: some concepts are clear:Related PD, “PD is an age-dependent neurodegenerative disease, characterized by progressive loss of midbrain dopaminergic neurons and formation of Lewy bodies. Although the pathogenesis of PD has not been completely elucidated, excessive accumulation of toxic forms of α-synuclein (α-syn) with a series of secondary pathological cascades plays crucial roles in the onset of PD. This is mainly due to an imbalance between production and clearance of α-syn in the brain. Related the literature reported is clear the function of some physiologic system inside brain that play a role in its omeostasys [17-19]. In animal model transgenic mouse “Findings suggest that lymphatic clearance dysfunction is crucial in the onset and development of PD”. And related GLYMPHATIC SYSTEM “the glymphatic system facilitates convective exchange of various interstitial solutes including glucose, lipids, amino acids, lactic acid and neurotransmitters between cerebrospinal fluid (CSF) and interstitial fluid (ISF)“. “Our results demonstrate an impairment of glymphatic clearance of α-syn in the brain of A53T mice, and disruption of meningeal lymphatic drainage further exacerbates α-syn-related pathology”.
But related other neurodegenerative disease like AD: “The present study shows that treatment with detox gel in AD mice can be effective as a therapeutic method for lowering Aβ levels in the APPSWE mice (Tg2546). The high affinity sequestering ability combined with the lack of immunogenicity and capacity to improve memory parameters make it an interesting drug candidate for the treatment of AD. Thus our detox gel therapy might become a welcome non-immune based therapy with a future in human clinical settings” and “Quantitative analysis of Aβ-42 in brain Aβ in brain was extracted by methods described in experimental methods section. Aβ-42 levels were measured in brain extracts by an ELISA using a commercial antibody. The two step extraction method that we employed yields total Aβ. The results show that there is a reduction in the level of total Aβ-42 by 30% in the group of mice that received the detox gel when compared to the untreated group with a statistical significance (p < 0.001)” novel Detox Gel Depot sequesters β-Amyloid Peptides in a mouse model of Alzheimer’s Disease”. “Dairy foods (milk, cheese) might be positively associated with increased risk of PD, especially for men“. “We demonstrate that a substantial portion of subarachnoid CSF cycles through the brain interstitial space. On the basis of in vivo two-photon imaging of small fluorescent tracers, we showed that CSF enters the parenchyma along paravascular spaces that surround penetrating arteries and that brain interstitial fluid is cleared along paravenous drainage pathways. Animals lacking the water channel aquaporin-4 (AQP4) in astrocytes exhibit slowed CSF influx through this system and a ~70% reduction in interstitial solute clearance, suggesting that the bulk fluid flow between these anatomical influx and efflux routes is supported by astrocytic water transport.
Fluorescent-tagged amyloid β, a peptide thought to be pathogenic in Alzheimer’s disease, was transported along this route, and deletion of the Aqp4 gene suppressed the clearance of soluble amyloid β, suggesting that this pathway may remove amyloid β from the central nervous system”. The glymphatic pathway expedites clearance of waste, including soluble amyloid β (Aβ) from the brain. Transport through this pathway is controlled by the brain’s arousal level because, during sleep or anesthesia, the brain’s interstitial space volume expands (compared with wakefulness), resulting in faster waste removal“. “The rodent brain removes waste better during sleep or anesthesia compared with the awake state“.
Related the result of this work is relevant for some neurodegeneretaive condition to verify the role played by brain glymphatic system as well as the effect played by some brain vascular inefficiencies and the role played by SOME TOXIC catabolic substantia and their accumulation. An imbalances between production and clearance of alfa-sinuclein in the brain is involved in PD evolution. The relationship with some kind of food and PD is not so high but what is to take in consideration is the functionality of the brain clearance system that can be more saturated is some kind of diet. Also interesting the way of influence in this system by body posture (is a dynamic process). Is possible that 2 factors influence this process?
Factor A: clearance efficiency of the system in basal status (genetic factor, age)
Factor B: Clearance efficiency in SATUTARED situation? (Environmental factor) and
Global Function: A x B
This results can be used to search new therapeutic strategies: or to reduce saturation condition of the system analyzed or new molecule that can improve the “washing” of this toxic endogenous substantial or a combination of this 2. A detox gel in mouse model of DA showed activity to suggest to develop this strategy, and the same observing epidemiological data related world PD incidence compared to some diet it seen to show how reduce the saturation of this system. As global conclusion of this work is possible to say that a combination of this 2 strategies can produce an interesting clinical effect to be verified. (This combined strategy is a new association: Pharmacological effect, added to de toxicant strategy). 4 are the main factor involved: The toxic molecule to be depurate, the carrier- vectors, the brain washing system, saturation condition (Environmental factor).
In animal model the detox strategy produced effect, so is possible to say that a toxicological approach can be a way to be walked. A “total Aβ-42 by 30% in the group of mice that received the detox gel when compared to the untreated group with a statistical significance (p < 0.001)” (5) is a good result to start. A new drug class able to link and detox the toxicological molecule and to be carried out from brain structure using the glimphatic system through adequate pharmacokinetics chemical group. So in drug design activity: Drugs with 2 parts: a part that link the toxico molecule (detox), added to another part with Pharmacokinetics group for brain glimphatic system. Molecule whin the necessary biotollerability, absence of toxicity and high kinetics properties (to arrive in site of actions and to be washed). High affinity, sequestering ability, not immunogenicity, right molecular weight, lipofilic idrofilic balances right electrical charges, persistence of actions and other relevant molecular properties drive in the research of this new durgs. BEE is a crucial protective structure of CNS since fron outside of it but it can be also crucial in the outside Transfer of toxic- cathabolic products. A deep knowledge in this dynamics make possible to search innovative approach in an endogen toxicological condition. Keyword to be investigated: brain wasting molecule clearance, kinetics of the process, genetic, environmental factors, saturation process. The finding in animal model are interesting point to start.
- Wenyan Z, Tinglin P, Weixi F, Ming L, Ying Z, et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Translational Neurodegeneration. 2019; 8: 7. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30867902
- Abdelrahman YF, Susan CF, Adviye E. Brain Vasculature and Cognition Renin-Angiotensin System, Endothelin and Beyond.
- Ranjini KS, Chinnaswamy K, Stanley S, Pazhani S. Novel Detox Gel Depot sequesters β-Amyloid Peptides in a mouse model of Alzheimer’s disease. Int J Pept Res Ther. 2012; 18: 99-106. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22712003
- Dong SK, Ho-Il Choi, Yun W, Yu L, Barry J. et al. A New Treatment Strategy for Parkinson's disease through the Gut–Brain Axis the Glucagon-Like Peptide-1 Receptor Pathway. Cell Transplant. 2017 Sep; 26(9): 1560-1571. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29113464
- Ling W, Qi Z, Huanbin L, Hong Z. SPECT Molecular Imaging in Parkinson's Disease. J Biomed Biotechnol. 2012; 2012: 412486. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22529704
- Jiang W, Ju C, Jiang H, Zhang D. Dairy foods intake and risk of Parkinson's disease: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2014; 29: 613-619. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24894826
- Stacey ES, Jose AS, Hope B, Judith AP. The emerging role of nutrition in Parkinson's disease. Front Aging Neurosci. 2014; 6: 36. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24639650
- Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, et al. Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov Disord. 1999; 14: 21-27. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9918340
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012; 4: 147ra111. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22896675
- Hedok L, Lulu X, Mei Y, Hongyi K, Tian F, et al. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015; 35: 11034-11044. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26245965
- Maiken N. Garbage Truck of the Brain. Science. 2013; 340: 1529-1530. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23812703
- Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004; 24: 1888-1896. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14985429
- Marques O, Outeiro TF. Alpha-synuclein: from secretion to dysfunction and death. Cell Death & Disease. 2012; 3.
- Bao LS, Li-hua W, Tuo Y, Jing-yi S, Lei-lei M, et al. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog Neurobiol. 2018; 118: 163-164. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28903061
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014; 34: 16180-16193. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25471560
- Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018; 17: 1016-1024.
- Luisetto M, Ibrahim G, Muhamad A, Areeba I, Behzad N. Brain Washing System-The System by Which the Wasting Molecules are removed from Brain. J Anat Pathol. 2019; 1: 1-5.
- Luisetto M, Naseer A, Behzad N, Gamal AH, Ghulam RM, et al. Endogenus Toxicology: Modern Physio- Pathological Aspects and Relationship with New Therapeutic Strategies. An Integrative Discipline Incorporating Concepts from Different Research Discipline like Biochemistry, Pharmacology and Toxicology. Arch Cancer Sci Ther. 2019; 3: 001-024.
- Bryan AK, Viviane L. Vertebrate food products as a potential source of prion-like α-synuclein. Parkinson’s disease. 2017; 3: 33. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29184902