Case Report
Direct Carotid Puncture for Flow Diverter Stent Insertion

Bhogal P1* Phillips TJ2 and Makalanda HLD1
1Department of Neuroradiology, The Royal London Hospital, ST. Bartholomew’s and the Royal London Hospital NHS Trust, London, UK
2Neurological Intervention & Imaging Service of Western Australia, First Floor G Block, Sir Charles Gairdner Hospital, Verdun St, Nedlands, Perth, 6009 Australia
*Address for Correspondence: Dr. P. Bhogal, Department of Neuroradiology, The Royal London Hospital, St. Bartholomew’s and the Royal London Hospital NHS Trust, London, UK, Tel: 00 44 7815 937220; Email: [email protected]
Dates: Submitted: 01 June 2017; Approved: 29 June 2017; Published: 30 June 2017
How to cite this article: Bhogal P, Phillips TJ, Makalanda HLD. Direct Carotid Puncture for Flow Diverter Stent Insertion. J Neurosci Neurol Disord. 2017; 1: 024-028. DOI: 10.29328/journal.jnnd.1001004
Copyright License: © 2017 Bhogal P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Aneurysm; Flow diverter; Stent; Carotid puncture
ABSTRACT
Objective: To report our experience of direct carotid puncture and its use in the management of a large and rapidly expanding cavernous aneurysm.
Methods: A patient with a cavernous aneurysm that measured 25mm in maximum diameter underwent treatment with flow diversion. The initial treatment strategy was parent vessel occlusion however she failed the balloon occlusion test at 3 minutes. Due to extremely tortuous vessels stable access via a common femoral artery approach was impossible to achieve. We present our strategy, the post-operative management and long term results.
Results: Using a direct carotid puncture three telescoped Pipeline embolisation devices were successfully deployed across the neck of the cavernous aneurysm without complication. The puncture site formed a stable platelet plug after direct compression with an ultrasound probe for 90 minutes with no post-operative complications either intracranially or at the neck puncture site. At 2 year follow-up the aneurysm is completely excluded from the circulation.
Conclusion: Direct carotid puncture can be used as access for intracranial interventional procedures even if patients are on dual anti-platelet medication.
INTRODUCTION
Endovascular procedures have increased in frequency and complexity in recent years. This increasing complexity has been in part due to the development of dedicated devices that allow access to the intracranial vasculature in cases of tortuous anatomy. Flexible catheters that provide stability during procedures is a pre-requisite for a safe and effective treatment however, in some instances this cannot be achieved via a common femoral approach. In these instances alternate routes of access need to be considered even though they may be unfamiliar particularly to junior interventionists. These alternate routes can include the radial, brachial, subclavian or common carotid arteries. In order to minimise complications a clear strategy is required not only for the puncture and procedure itself but also for the post-operative management. This is particularly true for direct carotid punctures and when patients are taking anti-platelet medications or anti-coagulants which is frequent in our patient population. Here we present a case of direct carotid puncture to deploy a flow diverting stent.
CASE REPORT
A 74-year-old female patient was being actively monitored for 10 years with a stable 5mm cavernous aneurysm. She represented at her annual check-up with a new 6th nerve palsy. Repeat MRA demonstrated that the aneurysm had rapidly expanded to 25mm in the intervening 12 months and referral to our department was made. She had no other comorbidity and was a non-smoker. After careful discussion about the relative benefits and risks of treatment the plan at a multidisciplinary meeting was made for catheter angiography and balloon test occlusion.
Catheter angiography proved challenging due to a type III aortic arch and tortuous vascular anatomy ascending from the right common femoral puncture site (Figure 1). Unfortunately she failed balloon test occlusion at 3 min both clinically and angiographically.
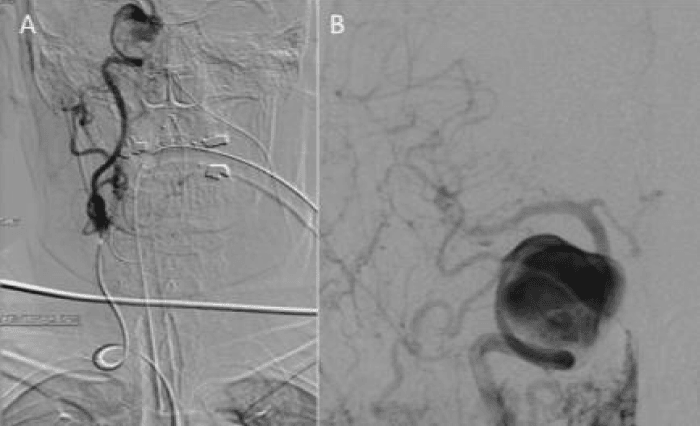
Figure 1: Catheter angiography required a Sim2 catheter and proved extremely difficult due to marked aortic tortuosity, a type III aortic arch and further tortuosity of the internal carotid artery (ICA) (Figure 1a). Despite this angiography via the common carotid artery did manage to successfully show a large aneurysm arising from the cavernous segment of the ICA (Figure 1b).
The plan was then made for flow diverter stent placement to treat the giant cavernous aneurysm and the patient commenced on dual antiplatelet medication for a week prior to the procedure. Given the difficulty at initial angiography, she was also consented for possible neck puncture. The procedure began with puncture of the right common femoral artery and under full heparinisation (5000IU) to maintain the ACT 2-2.5 times normal. Despite multiple attempts with a 70cm long sheath (Cook shuttle, Cordis) and multiple operators, the internal carotid could not be accessed via the femoral route. At this point carotid puncture was determined the next most feasible option. The catheter was left in the common carotid artery for roadmaps and check angiography. Under ultrasound guidance the common carotid artery was then punctured, using a single wall technique, with a micropuncture set (Cook Medical) and a 4Fr Brite tip sheath (Cordis) placed. The micropuncture guidewire was placed in the external carotid artery (Figure 2a) so as to minimise potential risks to the ICA.
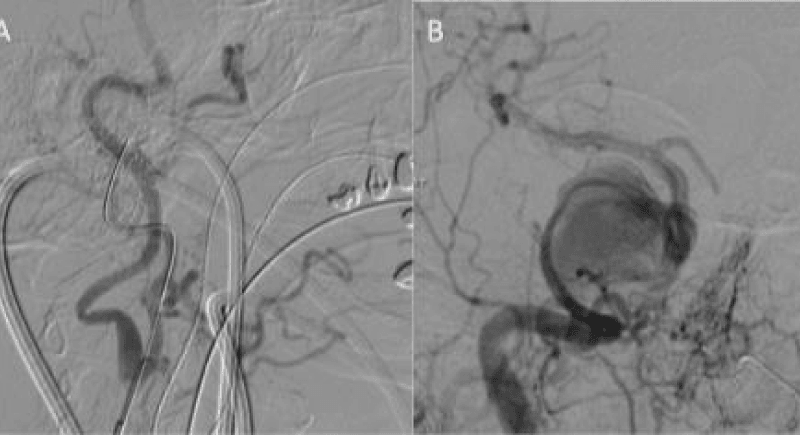
Figure 2: The common carotid artery was punctured using a micropuncture kit and under direct ultrasound guidance. The wire was initially tracked into the ECA to prevent damaging the ICA. The sheath was then tracked in the ICA with help from angiography via the common femoral catheter (Figure 2a). Once the 4Fr neck sheath was secure in the ICA a Marksman catheter was navigated into the M1 segment of the middle cerebral artery and 3 PED’s were deployed using a telescoping technique.
The neck sheath was then used for navigation of the Marksman catheter (Covidien) under roadmap from the groin sheath. After successful passage across the aneurysm (Figure 2b), three Pipeline Embolisation Devices (PED’s) (Covidien) were telescoped across the aneurysm with a good angiographic result (Figure 3). Our initial plan to jail a microcatheter in the aneurysm to place additional coils after the stent placement could not be performed due to the difficulties in accessing the aneurysm. An Angioseal (St. Jude Medical) was used for the groin closure. Manual compression of the carotid required 90 minutes of direct pressure with ultrasound to achieve haemostasis. The patient was left intubated overnight.
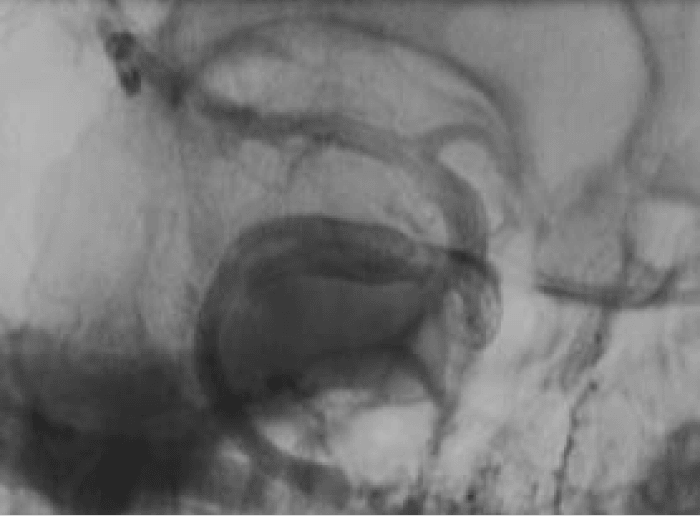
Figure 3: Angiography performed at the end of the procedure showed satisfactory position of the PED’s and stagnation of contrast in the aneurysm (not shown).
She was extubated uneventfully the following morning and was home day 2 post procedure. Despite two weeks of steroids her sixth nerve palsy progressed to a complete cavernous syndrome in the first 48 hours post procedure. Her symptoms improved six weeks post procedure however, she has a residual sixth nerve palsy. Imaging demonstrated complete occlusion of the aneurysm on both follow-up MRA and catheter angiogram at 6 months and 2 years (not shown). She remains well on lifelong aspirin.
DISCUSSION
A pre-requisite to performing any form of endovascular treatment within the intracranial vasculature is the ability to achieve and maintain a stable position. Tortuosity of the iliac arteries, aorta and supra-aortic vessels can make this extremely difficult and loss of guide catheter position can readily occur with some authors suggesting approximately 5% of endovascular treatments fail because of tortuous vascular anatomy [1]. The advancements in catheters and guide wires over the last decade have no doubt made access easier however, there will always be cases that are challenging. In these cases alternative routes of access need to be considered. Often the difficulties with achieving access via the standard femoral route will be encountered again if the radial or brachial route is attempted and it is worth noting that other conditions such as bilateral ilio-femoral arterial occlusion, thoracic aortic dissection or severe atherosclerosis can all result in similar difficulties with stable access. Therefore, in these select few cases the carotid route should be considered.
Access to the carotid artery can be achieved in one of two ways-a cut down with visualised direct puncture of the common carotid artery (CCA), or percutaneous puncture of the artery either with or without image guidance such as ultrasound or road map assistance from an in-dwelling catheter in the origin of the CCA. The cut down method was in fact advocated by the founding father of cerebral angiography, Egas Moniz and his colleague Almeida Lima would favour puncture of the CCA with ultrasound guidance as we feel this offers several advantages to the operator including clearly delineating the position of the internal jugular vein in relation to the CCA, the level of the bifurcation, ensuring a double wall puncture is not performed and the ability to avoid areas of atherosclerotic plaque that may otherwise embolise during the puncture.
In 2006 Moret and colleagues published their retrospective review of 42 (38 involving the CCA or ICA, and 4 involving the cervical vertebral artery) direct percutaneous punctures to treat a variety of conditions including aneurysms, meningiomas, AVM and carotid-cavernous fistula [2]. They reported a 100% success rate in accessing the intracranial target lesion via this route. Other groups have reported using the percutaneous technique to treat anterior circulation aneurysms [3], as well as carotid artery stenting [4,5] and recently the primary author was involved in two acute thrombectomy cases that required percutaneous carotid puncture.
Complications are obviously a major concern when using this technique. Moret et al. reported a 14% complication rate that included transient vasospasm, an asymptomatic aneurysm at the site of puncture, and cervical haematomas one of which required surgical evacuation as it compromised the airway. This latter complication is one of the most feared and is more likely to occur in the setting of anti-platelet and anti-coagulant use [2,3,6-8]. It is therefore advantageous to have airway control and consider a prolonged period of intubation to ensure a stable platelet plug has formed at the puncture site. Blanc et al have previously reported cervical haematomas were associated with extensive anticoagulation and/or anti-platelet treatment, or the use of sheaths larger than 6Fr [2]. For this reason we believe that it is advisable to perform anti-platelet testing to exclude hyper-responders from this technique.
We also prefer to use ultrasound when applying compression as this allows us to monitor the actual puncture site yet still maintain adequate blood flow to the brain which could otherwise be impeded through over-zealous manual compression. The use haemostatic closure devices has been reported in the setting of direct carotid puncture [2,4,9] and whilst there have been no reported complications from the use of these devices in this setting, complications from the use of vascular closure devices are well documented [10,12]. Therefore, we felt that in our hands compression under direct ultrasound observation represented the safest option to achieve haemostasis.
CONCLUSION
Direct carotid puncture remains a viable option under certain conditions but careful planning of each stage of the procedure is required in order to minimise the risk of complications. Direct carotid puncture should be considered a viable alternative access route not only for complicated procedures involving flow diverters but also more time pressured interventional procedures such as mechanical thrombectomy.
REFERENCES
- Aletich VA, Debrun GM, Misra M, Charbel F, Ausman JI. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg. 2000; 93: 388-396. Ref.: https://goo.gl/PuAqdw
- Blanc R, Piotin M, Mounayer C, Spelle L, Moret J. Direct cervical arterial access for intracranial endovascular treatment. Neuroradiology. 2006; 48: 925-929. Ref.: https://goo.gl/9k8njH
- Nii K, Kazekawa K, Onizuka M, Aikawa H, Tsutsumi M, et al. Direct Carotid Puncture for the Endovascular Treatment of Anterior Circulation Aneurysms. Am J Neuroradiol. 2006; 27: 1502-1504. Ref.: https://goo.gl/VMDZWU
- Guimaraens L, Theron J, Casasco A, Cuellar H. Carotid artery stenting by direct percutaneous puncture. J Vasc Surg. 2011; 54: 249-251. Ref.: https://goo.gl/XjMbct
- Perez-Arjona EA, Delprosto Z, Fessler RD. Direct percutaneous carotid artery stenting with distal protection: technical case report. Neurol Res. 2004; 26: 338-341. Ref.: https://goo.gl/NVRMSu
- Dorfer C, Standhardt H, Gruber A, Ferraz-Leite H, Knosp E, et al. Direct percutaneous puncture approach versus surgical cutdown technique for intracranial neuroendovascular procedures: technical aspects. World Neurosurg. 2012; 77: 192-200. Ref.: https://goo.gl/Mti6ai
- Takata H, Iida T, Akai T, Kumano K, Iizuka H, et al. Coil embolization for intracranial aneurysm by direct puncture of the carotid artery in elderly patients. Interv Neuroradiol. 1998; 4: 75-76. Ref.: https://goo.gl/1PHA57
- Yuzawa I, Kurata A, Suzuki S, Ozawa H, Hagiwara H, et al. Efficacy of a direct puncture approach for anterior circulation aneurysms using a newly developed guiding catheter - especially for geriatric patients. Surg Neurol. 2007; 67: 30-34. Ref.: https://goo.gl/fdjuoy
- Blanc R, Mounayer C, Piotin M, Sadik JC, Spelle L, et al. Hemostatic closure device after carotid puncture for stent and coil placement in an intracranial aneurysm: technical note. AJNR Am J Neuroradiol. 2002; 23: 978-981. Ref.: https://goo.gl/cfgSq2
- Modi S, Gadvi R, Babu S. Initial experience with AngiosealTM: Safety and efficacy of the endovascular closure device. Indian J Radiol Imaging. 2013; 23: 134-138. Ref.: https://goo.gl/aaaMVv
- Corley JA, Kasliwal MK, Tan LA, Lopes DK. Delayed Vascular Claudication Following Diagnostic Cerebral Angiography: A Rare Complication of the AngioSeal Arteriotomy Closure Device. J Cerebrovasc Endovasc Neurosurg. 2014; 16: 275-280. Ref.: https://goo.gl/nN5b8v
- Lewis-Carey MB, Kee ST. Complications of arterial closure devices. Tech Vasc Interv Radiol. 2003; 6: 103-106. Ref.: https://goo.gl/QSyVa6