Short Communication
The “Calcium Paradox” Due To Ca2+/Camp Interaction: New Insights for the Neuroscience Field

Leandro Bueno Bergantin* and Afonso Caricati-Neto
Laboratory of Autonomic and Cardiovascular Pharmacology, Department of Pharmacology, Escola Paulista de Medicina, Universidade Federal de São Paulo, Brazil
*Address for Correspondence: Leandro Bueno Bergantin, Laboratory of Autonomic and Cardiovascular Pharmacology, Department of Pharmacology, Escola Paulista de Medicina, Universidade Federal de São Paulo (UNIFESP), 55 11 5576-4973, Rua Pedro de Toledo, 669-Vila Clementino, São Paulo-SP, Brazil, Tel: 04039-032; Email: [email protected]
Dates: Submitted: 25 January 2017; Approved: 17 February 2017; Published: 21 February 2017
How to cite this article: Bergantin LB, Caricati-Neto A. The “Calcium Paradox” Due To Ca2+/Camp Interaction: New Insights for the Neuroscience Field. J Neurosci Neurol Disord. 2017; 1: 012-015. DOI: 10.29328/journal.jnnd.1001002
Copyright License: © 2017 Bergantin LB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ca2+/cAMP interaction; “Calcium paradox”; Neurodegenerative diseases
ABSTRACT
In the cardiovascular field, tachycardia and increment of catecholamine plasma levels (sympathetic hyperactivity) have been reported by hypertensive patients that use L-type Ca2+ channel blockers (CCBs) since 70´s. Our discovery of the involvement of interaction between the intracellular signalling pathways mediated by Ca2+ and cAMP (Ca2+/cAMP interaction) revealed that this phenomenon (sympathetic hyperactivity) was resulting of increase of transmitter release from sympathetic neurons stimulated by CCBs due to its interference on the Ca2+/cAMP interaction. In the neuroscience field, this discovery has produced new paths for the understanding of the cellular and molecular mechanisms involved in the pathogenesis of neurodegenerative diseases, such as Alzheimer´s and Parkinson’s diseases. In this way, novel journeys for the development of new pharmacological strategies more effective for the treatment of neurodegenerative diseases may be initiated.
INTRODUCTION
Many results have shown that cAMP increases neurotransmitter release at many synapses in autonomic nervous system of vertebrate, including sympathetic neurons [1]. The notion of stimulus-secretion initially resulted from the experiments performed by Douglas and Rubin in the 1960s [2]. Using adrenal chromaffin cells, Baker and Knight revealed in 1970´s that a rise in the cytosolic Ca2+ concentration ([Ca2+] c) is an elementary requirement to trigger transmitter release [3]. The demonstration of direct relationship between rapid neurotransmitter release and rise in [Ca2+]c derived from the experiments using photo released caged Ca2+ in adrenal chromafin cells performed Neher and Zucker in 1990´s [4]. Although the cellular and molecular mechanisms involved in these synergistic effects of cAMP on the release of neurotransmitter and hormones are indistinct, the evidences suggest that this important intracellular messenger modulates intracellular signalling mediated by Ca2+ involved in the regulation of neurotransmitter, and hormones release.
The ca2+/camp interaction hypothesis
The hypothesis for a suitable interaction between the intracellular signalling pathways mediated by Ca2+ and cAMP, named Ca2+/cAMP interaction, has been widely studied in different cell types and tissues. The Ca2+/cAMP interaction has particularly been extensively studied at the endoplasmic reticulum (ER) Ca2+ channels, such as Ca2+ channels regulated by ryanodine receptors (RyR) [5-8]. In general, this interaction results in synergistic actions of these intracellular messengers on cell functions regulated by adenylyl cyclases (ACs), or phosphodiesterases (PDEs) [5-8]. Indeed, Ca2+/cAMP interaction plays a role in neurotransmitter release from neurons and neuroendocrine cells [5-8]. Then, novel therapeutic insights for medicines could be developed by the pharmacological modulation of the Ca2+/cAMP interaction.
The ca2+/camp interaction: new insights for neuroscience
Sympathetic hyperactivity such as tachycardia, and increment of catecholamine plasma levels, have been evidenced by several medical studies dealing with CCBs [9]. Despite these adverse effects of CCBs have been initially attributed to adjust reflex of arterial pressure, during almost four decades the cellular and molecular mechanisms involved this enigmatic phenomenon named “calcium paradox” remained unclear.
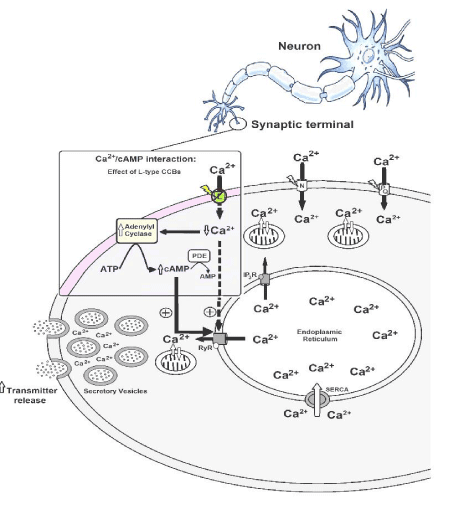
In 2013, we revealed that “calcium paradox” phenomenon resulted from the increment of transmitter release from sympathetic neurons, and adrenal chromaffin cells, stimulated by CCBs due to its interference on the Ca2+/cAMP interaction [6]. Using isolated tissues richly innervated by sympathetic nerves (rat vas deferens) to exclude the influence of adjusting reflex, we showed that neurogenic responses of the vas deferens were completely inhibited by L-type CCBs in high concentrations (>1 μmol/L), but unpredictably, and paradoxically, potentiated in concentrations below 1 μmol/L, characterized by sympathetic hyperactivity induced by CCBs [10-12]. Indeed, this paradoxical sympathetic hyperactivity is caused by increment of neurotransmitter release from sympathetic neurons due to its pharmacological modulation on the Ca2+/cAMP interaction [5-8] (Figure 1).
Figure 1: Increase of the neurotransmitter release due to pharmacological modulation of the Ca2+/cAMP interaction by L-type CCBs [5-8].
Undeniably, several studies showed that neuroprotective response can be achieved by increase of cytosolic cAMP concentration ([cAMP] c) stimulation [13,14]. In this way, we could propose that a rise of [cAMP]c by interfering in the Ca2+/cAMP interaction could attenuate neuronal death triggered by cytosolic Ca2+ overload [5-8,15,16]. Then, the pharmacological modulation of the Ca2+/cAMP interaction [17,18] produced by combination of the L-type CCBs used in the antihypertensive therapy, and [cAMP]c enhancer compounds used in the anti-depressive therapy such as rolipram, could be a new pharmacological strategy for enhancing neurotransmission in neurological and psychiatric disorders [19,20] resulting from neurotransmitter release deficit, and/or neuronal death [5-8,15,16]. These results could open a new path for the drug development more effective and safer for the treatment of neurodegenerative diseases.
CONCLUSION
New insights for the neuroscience field from the discovery of the “calcium paradox” due to Ca2+/cAMP interaction have been emerging to treat neurodegenerative diseases. Pharmacological modulation of this interaction could be a more efficient and safer therapeutic strategy for stimulating neurotransmission compromised by neurotransmitter release deficit, and attenuating neuronal death in the neurodegenerative diseases.
DISCLOSURE STATEMENT
Caricati-Neto and Bergantin thank the continued financial support from CAPES, CNPq and FAPESP (Bergantin´s Postdoctoral Fellowship FAPESP #2014/10274-3).
The authors also thank Elsevier - “author use”:
Reuse of portions or extracts from the article in other works- https://www.elsevier.com/__data/assets/pdf_file/0007/55654/AuthorUserRights.pdf
REFERENCES
- Chern YJ, Kim KT, Slakey LL, Westhead EW. Adenosine receptors activate adenylate cyclase and enhance secretion from bovine adrenal chromaffin cells in the presence of forskolin. J Neurochem. 1988; 50: 1484-1493. Ref.: https://goo.gl/D3U5F0
- Douglas WW, Rubin RP. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961; 159: 40-57. Ref.: https://goo.gl/Lwekpm
- Baker PF, Knight DE. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978; 276: 620-622. Ref.: https://goo.gl/bvf8DR
- Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993; 10: 21-30. Ref.: https://goo.gl/3fvzjy
- Caricati-Neto A, García AG, Bergantin LB. Pharmacological implications of the Ca2+/cAMP signalling interaction: from risk for antihypertensive therapy to potential beneficial for neurological and psychiatric disorders. Pharmacol Res Perspect. 2015; 3: e00181. Ref.: https://goo.gl/UiQrGs
- Bergantin LB, Souza CF, Ferreira RM, Smaili SS, Jurkiewicz NH, et al. Novel model for “calcium paradox” in sympathetic transmission of smooth muscles: role of cyclic AMP pathway. Cell Calcium. 2013; 54: 202-212. Ref.: https://goo.gl/HHou6F
- Bergantin LB, Jurkiewicz A, García AG, Caricati-Neto A. A Calcium Paradox in the Context of Neurotransmission. Journal of Pharmacy and Pharmacology. 2015; 3: 253-261. Ref.: https://goo.gl/ltkVhv
- Bergantin LB, Caricati-Neto A. Challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca2+/cAMP intracellular signalling interaction. Eur J Pharmacol. 2016; 788: 255-260. Ref.: https://goo.gl/u21xBi
- Grossman E, Messerli FH. Effect of calcium antagonists on sympathetic activity. Eur Heart J. 1998; 19: F27-F31. Ref.: https://goo.gl/BQkkG1
- Kreye VA, Luth JB. Proceedings: verapamil-induced phasic contractions of the isolated rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1975; 287: R43. Ref.: https://goo.gl/Z07NA4
- French AM, Scott NC. A comparison of the effects of nifedipine and verapamil on rat vas deferens. Br J Pharmacol. 1981; 73: 321-323. Ref.: https://goo.gl/d8wrcd
- Moritoki H, Iwamoto T, Kanaya J, Maeshiba Y, Ishida Y, et al. Verapamil enhances the non-adrenergic twitch response of rat vas deferens. Eur J Pharmacol. 1987; 140: 75-83. Ref.: https://goo.gl/OKoviM
- Sommer N, Loschmann PA, Northoff GH, Weller M, Steinbrecher A, et al. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat Med. 1995; 1: 244-248. Ref.: https://goo.gl/MiganR
- Xiao L, O'Callaghan JP, O'Donnell JM. Effects of repeated treatment with phosphodiesterase-4 inhibitors on cAMP signaling, hippocampal cell proliferation, and behavior in the forced-swim test. J Pharmacol Exp Ther. 2011; 338: 641-647. Ref.: https://goo.gl/14xO4A
- Bergantin LB, Caricati-Neto A. Novel Insights for Therapy of Parkinson’s disease: Pharmacological Modulation of the Ca2+/cAMP Signalling Interaction. Austin Neurol & Neurosci. 2016; 1: 1009. Ref.: https://goo.gl/ujivdj
- Bergantin LB, Caricati-Neto A. Recent advances in pharmacotherapy of neurological and psychiatric disorders promoted by discovery of the role of Ca2+/cAMP signaling interaction in the neurotransmission and neuroprotection. Adv Pharmac J. 2016; 1: 66.
- Halls ML, Cooper DM. Adenylyl cyclase signalling complexes - Pharmacological challenges and opportunities. Pharmacol Ther. 2017; 7258: 30011-30016. Ref.: https://goo.gl/VDeVKV
- Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995; 374: 421-424. Ref.: https://goo.gl/OVc1JP
- Bereczki E, Francis PT, Howlett D, Pereira JB, Höglund K, et al. Synaptic proteins predict cognitive decline in Alzheimer`s disease and Lewy body dementia. Alzheimers Dement. 2016; 12: 1149-1158. Ref.: https://goo.gl/pch8Cq
- Clare R, King VG, Wirenfeldt M, Vinters HV. Synapse loss in dementias. J Neurosci Res. 2010; 88: 2083-2090. Ref.: https://goo.gl/5sJRu7