Abstract
Review Article
Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience Research
Alex, Gideon S*, Olanrewaju Oluwaseun Oke, Joy Wilberforce Ekokojde, Tolulope Judah Gbayisomore, Martina C. Anene-Ogbe, Farounbi Glory and Joshua Ayodele Yusuf
Published: 12 November, 2024 | Volume 8 - Issue 2 | Pages: 106-114
Background: The study of new neuron formation in the adult brain has sparked controversy and ignited interest among scientists in recent times, these include its occurrence and location in the adult human brain, functional significance, variation in study methods, translation from animal model to human, and ethical challenges involving neural stem cell research.
Aim: To provide a comprehensive understanding of adult neurogenesis, functional significance, and challenges and explore the latest advances in the study of adult neurogenesis.
Methodology: An extensive and systematic search of electronic databases (Medline, Scopus, Web of Science) was conducted using keywords related to adult neurogenesis and techniques involved in its study.
Results: The mechanism of adult neurogenesis was found to occur in specific brain regions such as the subgranular zone of the dentate gyrus and subventricular zone of the lateral ventricle. Adult neurogenesis is vital neural plasticity, providing a potential mechanism for the brain to adapt and reorganize in response to environmental cues and experiences. Cutting-edge research and sophisticated imaging techniques, such as two-photon microscopy, MRI, optogenetic, and stem-cell-based therapies have provided deeper insight into the study of adult neurogenesis.
Conclusion: The study of neurogenesis is important for understanding nervous system development, physiology, pathology, and exploring neuroplasticity. Its advancement is challenged by some ethical concerns regarding embryonic, pluripotent stem cells, and the need for safe, and noninvasive study methods. Although recent breakthroughs in neuroimaging, microscopic techniques, and genetic tools are aiding real-time study of adult neurogenesis.
Read Full Article HTML DOI: 10.29328/journal.jnnd.1001102 Cite this Article Read Full Article PDF
Keywords:
Adult neurogenesis; Neural stem cell; Neuroplasticity; Emerging technologies
References
- Moreno-Jiménez EP, Terreros-Roncal J, Flor-García M, Rábano A, Llorens-Martín M. Evidences for adult hippocampal neurogenesis in humans. J Neurosci. 2021;41(12):2541-2553. Available from: https://doi.org/10.1523/jneurosci.0675-20.2020
- Denoth-Lippuner A, Jessberger S. Formation and integration of new neurons in the adult hippocampus. Nat Rev Neurosci. 2021;22(4):223-236. Available from: https://doi.org/10.1038/s41583-021-00433-z
- Lieberwirth C, Pan Y, Liu Y, Zhang Z, Wang Z. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016;1644:127-140. Available from: https://doi.org/10.1016/j.brainres.2016.05.015
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313-1317. Available from: https://doi.org/10.1038/3305
- Horgusluoglu E, Nudelman K, Nho K, Saykin AJ. Adult neurogenesis and neurodegenerative diseases: a systems biology perspective. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):93-112. Available from: https://doi.org/10.1002/ajmg.b.32429
- Kuhn HG, Toda T, Gage FH. Adult hippocampal neurogenesis: a coming-of-age story. J Neurosci. 2018;38(49):10401-10410. Available from: https://doi.org/10.1523/jneurosci.2144-18.2018
- Kempermann G. Adult neurogenesis: an evolutionary perspective. Cold Spring Harb Perspect Biol. 2016;8(2). Available from: https://doi.org/10.1101/cshperspect.a018986
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310(5748):679-683. Available from: https://doi.org/10.1126/science.1115360
- Egeland M, Zunszain PA, Pariante CM. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat Rev Neurosci. 2015;16(4):189-200. Available from: https://doi.org/10.1038/nrn3855
- Choe Y, Pleasure SJ, Mira H. Control of adult neurogenesis by short-range morphogenic-signaling molecules. Cold Spring Harb Perspect Biol. 2016;8(3):a018887. Available from: https://doi.org/10.1101/cshperspect.a018887
- Bowman AN, Van Amerongen R, Palmer TD, Nusse R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin–responsive neural stem cells. Proc Natl Acad Sci U S A. 2013;110(18):7324-7329. Available from: https://doi.org/10.1073/pnas.1305411110
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131(12):2791-801. Available from: https://doi.org/10.1242/dev.01165
- Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J Neurosci. 2011;31(5):1676-1687. Available from: https://doi.org/10.1523/jneurosci.5404-10.2011
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long-term potentiation. J Biol Chem. 2006;281(17):11910-11916. Available from: https://doi.org/10.1074/jbc.m511920200
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311(1):223-237. Available from: https://doi.org/10.1016/j.ydbio.2007.08.038
- Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized β-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol. 2007;309(2):285-297. Available from: https://doi.org/10.1016/j.ydbio.2007.07.013
- Panchision DM, McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12(4):478-487. Available from: https://doi.org/10.1016/s0959-437x(02)00329-5
- Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11(7):493-501. Available from: https://doi.org/10.1038/nrc3079
- Li G, Hidalgo A. Adult neurogenesis in the Drosophila brain: the evidence and the void. Int J Mol Sci. 2020;21(18):6653. Available from: https://doi.org/10.3390/ijms21186653
- Fernández-Hernández I, Rhiner C, Moreno E. Adult neurogenesis in Drosophila. Cell Rep. 2013;3(6):1857-1865. Available from: https://doi.org/10.1016/j.celrep.2013.05.034
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149(1):134-148. Available from: https://doi.org/10.1016/0012-1606(92)90270-q
- Kato K, Awasaki T, Ito K. Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development. 2009;136(1):51-59. Available from: https://doi.org/10.1242/dev.023366
- Li G, Forero MG, Wentzell JS, Durmus I, Wolf R, Anthoney NC, et al. A Toll-receptor map underlies structural brain plasticity. Elife. 2020;9. Available from: https://doi.org/10.7554/elife.52743
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598-602. Available from: https://doi.org/10.1126/science.1248903
- Jacobs BL, Van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5(3):262-269. Available from: https://doi.org/10.1038/sj.mp.4000712
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978-981. Available from: https://doi.org/10.1126/science.271.5251.978
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687-702. Available from: https://doi.org/10.1016/j.neuron.2011.05.001
- Abdissa D, Hamba N, Gerbi A. Review article on adult neurogenesis in humans. Transl Res Anat. 2020;20:100074. Available from: https://doi.org/10.1016/j.tria.2020.100074
- Sierra A, Encinas JM, Maletic-Savatic M. Adult human neurogenesis: from microscopy to magnetic resonance imaging. Front Neurosci. 2011;5:47. Available from: https://doi.org/10.3389/fnins.2011.00047
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564-12568. Available from: https://doi.org/10.1073/pnas.0605177103
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133-143. Available from: https://doi.org/10.1016/j.cell.2005.04.028
- Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680-8685. Available from: https://doi.org/10.1523/jneurosci.1731-05.2005
- Novotny E, Ashwal S, Shevell M. Proton magnetic resonance spectroscopy: an emerging technology in pediatric neurology research. Pediatr Res. 1998;44(1):1-10. Available from: https://doi.org/10.1203/00006450-199807000-00001
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318(5852):980-985. Available from: https://doi.org/10.1126/science.1147851
- Riddle DR, Lichtenwalner RJ. Neurogenesis in the adult and aging brain. Brain Aging. 2007:127-158. Available from: https://www.ncbi.nlm.nih.gov/books/NBK3874/
- Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24(1):67-87. Available from: https://doi.org/10.1038/s41380-018-0036-2
- Mizuta K, Sato M. Multiphoton imaging of hippocampal neural circuits: techniques and biological insights into region-, cell-type-, and pathway-specific functions. Neurophotonics. 2024;11(3):033406. Available from: https://doi.org/10.1117/1.nph.11.3.033406
- Chen L, Cummings KA, Mau W, Zaki Y, Dong Z, Rabinowitz S, et al. The role of intrinsic excitability in the evolution of memory: Significance in memory allocation, consolidation, and updating. Neurobiol Learn Mem. 2020;173:107266. Available from: https://doi.org/10.1016/j.nlm.2020.107266
- Abrous DN, Wojtowicz JM. Interaction between neurogenesis and hippocampal memory system: new vistas. Cold Spring Harb Perspect Biol. 2015;7(6). Available from: https://doi.org/10.1101/cshperspect.a018952
- Yau SY, Li A, So KF. Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast. 2015;2015:717958. Available from: https://doi.org/10.1155/2015/717958
- Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat Rev Neurosci. 2017;18(6):335-346. Available from: https://doi.org/10.1038/nrn.2017.45
- Palanisamy CP, Pei J, Alugoju P, Anthikapalli NV, Jayaraman S, Veeraraghavan VP, et al. New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics. 2023;13(12):4138-4165. Available from: https://doi.org/10.7150/thno.83066
- Culig L, Chu X, Bohr VA. Neurogenesis in aging and age-related neurodegenerative diseases. Ageing Res Rev. 2022;78:101636. Available from: https://doi.org/10.1016/j.arr.2022.101636
- Kim MY, Kim MJ, Lee C, Lee J, Kim SS, Hong S, et al. Trametinib activates endogenous neurogenesis and recovers neuropathology in a model of Alzheimer’s disease. Exp Mol Med. 2023;55(10):2177-2189. Available from: https://doi.org/10.1038/s12276-023-01073-2
- Jastrzębski MK, Wójcik P, Stępnicki P, Kaczor AA. Effects of small molecules on neurogenesis: neuronal proliferation and differentiation. Acta Pharm Sin B. 2023 Oct 20. [Epub ahead of print]. Available from: https://doi.org/10.1016/j.apsb.2023.10.015
- Liu Y, Chu JM, Yan T, Zhang Y, Chen Y, Chang RC, et al. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice. J Neuroinflammation. 2020;17:4. Available from: https://doi.org/10.1186/s12974-019-1653-7
- Özbeyli D, Sarı G, Özkan N, Karademir B, Yüksel M, Kaya ÖT, et al. Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav Brain Res. 2017;328:159-77. Available from: https://doi.org/10.1016/j.bbr.2017.03.044
- Martini F, Régis Leite M, Gonçalves Rosa S, Pregardier Klann I, Wayne Nogueira C. Strength exercise suppresses STZ-induced spatial memory impairment and modulates BDNF/ERK-CAMKII/CREB signaling pathway in the hippocampus of mice. Cell Biochem Funct. 2020;38(2):213-221. Available from: https://doi.org/10.1002/cbf.3470
- Farzi MA, Sadigh-Eteghad S, Ebrahimi K, Talebi M. Exercise improves recognition memory and acetylcholinesterase activity in the beta amyloid-induced rat model of Alzheimer’s disease. Ann Neurosci. 2019;25(3):121-125. Available from: https://doi.org/10.1159/000488580
- Bonanni R, Cariati I, Tarantino U, D’Arcangelo G, Tancredi V. Physical exercise and health: a focus on its protective role in neurodegenerative diseases. J Funct Morphol Kinesiol. 2022;7(2):38. Available from: https://doi.org/10.3390/jfmk7020038
- Latchney SE, Eisch AJ. Therapeutic application of neural stem cells and adult neurogenesis for neurodegenerative disorders: regeneration and beyond. Eur J Neurodegener Dis. 2012;1(3):335-51. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4340249/
- Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8(9). Available from: https://doi.org/10.1101/cshperspect.a019026
- Apple DM, Fonseca RS, Kokovay E. The role of adult neurogenesis in psychiatric and cognitive disorders. Brain Res. 2017;1655:270-276. Available from: https://doi.org/10.1016/j.brainres.2016.01.023
- Alonso M, Petit AC, Lledo PM. The impact of adult neurogenesis on affective functions: of mice and men. Mol Psychiatry. 2024;29(8):2527-2542. Available from: https://doi.org/10.1038/s41380-024-02504-w
- Campbell S, MacQueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417-426. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC524959/
- Cui L, Li S, Wang S, Wu X, Liu Y, Yu W, et al. Major depressive disorder: hypothesis, mechanism, prevention, and treatment. Sig Transduct Target Ther. 2024;9:30. Available from: https://doi.org/10.1038/s41392-024-01738-y
- Mumtaz F, Khan MI, Zubair M, Dehpour AR. Neurobiology and consequences of social isolation stress in animal model—a comprehensive review. Biomed Pharmacother. 2018;105:1205-1222. Available from: https://doi.org/10.1016/j.biopha.2018.05.086
- Petković A, Chaudhury D. Encore: behavioural animal models of stress, depression, and mood disorders. Front Behav Neurosci. 2022;16:931964. Available from: https://doi.org/10.3389/fnbeh.2022.931964
- Dieni CV, Gonzalez JC, Overstreet-Wadiche L. Multifaceted circuit functions of adult-born neurons. F1000Res. 2019;8. Available from: https://doi.org/10.12688/f1000research.20642.1
- Ghosh HS. Adult neurogenesis and the promise of adult neural stem cells. J Exp Neurosci. 2019;13:1179069519856876. Available from: https://doi.org/10.1177/1179069519856876
- Jones KL, Zhou M, Jhaveri DJ. Dissecting the role of adult hippocampal neurogenesis towards resilience versus susceptibility to stress-related mood disorders. npj Sci Learn. 2022;7(1):16. Available from: https://doi.org/10.1038/s41539-022-00133-y
- Lampada A, Taylor V. Notch signaling as a master regulator of adult neurogenesis. Front Neurosci. 2023 Jun 29;17:1179011. Available from: https://doi.org/10.3389/fnins.2023.1179011
- Hussain G, Akram R, Anwar H, Sajid F, Iman T, Han HS, et al. Adult neurogenesis: a real hope or a delusion?. Neural Regen Res. 2024;19(1):6-15. Available from: https://doi.org/10.4103/1673-5374.375317
- Tsujimura K, Shiohama T, Takahashi E. microRNA biology on brain development and neuroimaging approach. Brain Sci. 2022;12(10):1366. Available from: https://doi.org/10.3390/brainsci12101366
- Mohammed OA, Elballal MS, El-Husseiny AA, Khidr EG, El Tabaa MM, Elazazy O, et al. Unraveling the role of miRNAs in the diagnosis, progression, and therapeutic intervention of Parkinson’s disease. Pathol Res Pract. 2023:155023. Available from: https://doi.org/10.1016/j.prp.2023.155023
- Kim TA, Syty MD, Wu K, Ge S. Adult hippocampal neurogenesis and its impairment in Alzheimer’s disease. Zool Res. 2022 May 5;43(3):481-96. Available from: https://doi.org/10.24272/j.issn.2095-8137.2021.479
- Phillips C. Lifestyle modulators of neuroplasticity: how physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plast. 2017;2017(1):3589271. Available from: https://doi.org/10.1155/2017/3589271
- Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. 2018;9:509. Available from: https://doi.org/10.3389/fpsyg.2018.00509
- Hullinger R, O’Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126-34. Available from: https://doi.org/10.1016/j.nlm.2015.08.006
- Zentall TR. Effect of environmental enrichment on the brain and on learning and cognition by animals. Animals. 2021 Mar 31;11(4):973. Available from: https://doi.org/10.3390/ani11040973
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12-21. Available from: https://doi.org/10.1016/j.expneurol.2011.01.008
- Ramírez-Rodríguez GB, Vega-Rivera NM, Meneses-San Juan D, Ortiz-López L, Estrada-Camarena EM, Flores-Ramos M. Short daily exposure to environmental enrichment, fluoxetine, or their combination reverses deterioration of the coat and anhedonia behaviors with differential effects on hippocampal neurogenesis in chronically stressed mice. Int J Mol Sci. 2021;22(20):10976. Available from: https://doi.org/10.3390/ijms222010976
- Llorente V, Velarde P, Desco M, Gómez-Gaviro MV. Current understanding of the neural stem cell niches. Cells. 2022;11(19):3002. Available from: https://doi.org/10.3390/cells11193002
- Liu B, Li Y, Ren M, Li X. Targeted approaches to delineate neuronal morphology during early development. Front Cell Neurosci. 2023;17:1259360. Available from: https://doi.org/10.3389/fncel.2023.1259360
- Hassan AU, Hassan G, Rasool Z. Role of stem cells in treatment of neurological disorder. Int J Health Sci. 2009 Jul;3(2):227-33. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3068820/
- Pinosanu LR, Wolff N, Olaru DG, Popa-Wagner A. Stem cell treatments in preclinical relevant stroke models. Curr Health Sci J. 2023;49(4):487-494. Available from: https://doi.org/10.12865/chsj.49.04.02
- Nalamolu KR, Chelluboina B, Fornal CA, Challa SR, Pinson DM, Wang DZ, et al. Stem cell treatment improves post stroke neurological outcomes: a comparative study in male and female rats. Stroke Vasc Neurol. 2021;6(4):519-527. Available from: https://doi.org/10.1136/svn-2020-000834
- Sadanandan N, Saft M, Gonzales-Portillo B, Borlongan CV. Multipronged attack of stem cell therapy in treating the neurological and neuropsychiatric symptoms of epilepsy. Front Pharmacol. 2021;12:596287. Available from: https://doi.org/10.3389/fphar.2021.596287
- de Oliveira CL, Bolzan JA, Surget A, Belzung C. Do antidepressants promote neurogenesis in adult hippocampus? A systematic review and meta-analysis on naive rodents. Pharmacol Ther. 2020;210:107515. Available from: https://doi.org/10.1016/j.pharmthera.2020.107515
Figures:
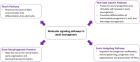
Figure 1

Figure 2
Similar Articles
-
Immunohistochemical expression of Nestin as Cancer Stem Cell Marker in gliomasRasha Mokhtar Abdelkareem*,Afaf T Elnashar,Khaled Nasser Fadle,Eman MS Muhammad. Immunohistochemical expression of Nestin as Cancer Stem Cell Marker in gliomas. . 2019 doi: 10.29328/journal.jnnd.1001027; 3: 162-166
-
Neurotoxicity related exposure to ambient nanoparticlesMojtaba Ehsanifar*,Zeinab Montazeri,Mehravar Rafati. Neurotoxicity related exposure to ambient nanoparticles. . 2022 doi: 10.29328/journal.jnnd.1001060; 6: 005-010
-
Improvement of the Cognitive Abilities in a Chronic Generalized Anxiety Disorder and Moderate Depression Case using a Novel Integrated Approach: The Cognitome ProgramMohita Shrivastava*. Improvement of the Cognitive Abilities in a Chronic Generalized Anxiety Disorder and Moderate Depression Case using a Novel Integrated Approach: The Cognitome Program. . 2024 doi: 10.29328/journal.jnnd.1001100; 8: 069-089
-
Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience ResearchAlex, Gideon S*,Olanrewaju Oluwaseun Oke,Joy Wilberforce Ekokojde,Tolulope Judah Gbayisomore,Martina C. Anene-Ogbe,Farounbi Glory,Joshua Ayodele Yusuf. Adult Neurogenesis: A Review of Current Perspectives and Implications for Neuroscience Research. . 2024 doi: 10.29328/journal.jnnd.1001102; 8: 106-114
Recently Viewed
-
Assessment of Perceptions of Nursing Undergraduates towards Mental Health PracticesAlya Algamdii*. Assessment of Perceptions of Nursing Undergraduates towards Mental Health Practices. Clin J Nurs Care Pract. 2025: doi: 10.29328/journal.cjncp.1001059; 9: 007-011
-
Multipurpose Antioxidants based on Food Industry Waste: Production and Properties EvaluationToshkhodjaev*. Multipurpose Antioxidants based on Food Industry Waste: Production and Properties Evaluation. Arch Food Nutr Sci. 2025: doi: 10.29328/journal.afns.1001062; 9: 001-003
-
Relationship between Fertility Diet Score Index Items and Ovulation in Women with Polycystic Ovary Syndrome: A Narrative ReviewHadis Alimoradi,Faezeh Mashhadi,Ava Hemmat,Mohsen Nematy,Maryam Khosravi,Maryam Emadzadeh,Nayere Khadem Ghaebi,Fatemeh Roudi*. Relationship between Fertility Diet Score Index Items and Ovulation in Women with Polycystic Ovary Syndrome: A Narrative Review. Arch Food Nutr Sci. 2024: doi: 10.29328/journal.afns.1001061; 8: 041-048
-
Evaluation of the LumiraDx SARS-CoV-2 antigen assay for large-scale population testing in SenegalMoustapha Mbow*,Ibrahima Diallo,Mamadou Diouf,Marouba Cissé#,Moctar Gningue#,Aminata Mboup,Nafissatou Leye,Gora Lo,Yacine Amet Dia,Abdou Padane,Djibril Wade,Josephine Khady Badiane,Oumar Diop,Aminata Dia,Ambroise Ahouidi,Doudou George Massar Niang,Babacar Mbengue,Maguette Dème Sylla Niang,Papa Alassane Diaw,Tandakha Ndiaye Dieye,Badara Cisé,El Hadj Mamadou Mbaye,Alioune Dieye,Souleymane Mboup. Evaluation of the LumiraDx SARS-CoV-2 antigen assay for large-scale population testing in Senegal. Int J Clin Virol. 2022: doi: 10.29328/journal.ijcv.1001041; 6: 001-006
-
The Use and Prevalence of Cannabis among Students of Nnamdi Azikiwe University, Awka, Anambra StateChijioke M Ofomata*,Enyinna P Nnabuihe. The Use and Prevalence of Cannabis among Students of Nnamdi Azikiwe University, Awka, Anambra State. J Forensic Sci Res. 2025: doi: 10.29328/journal.jfsr.1001088; 9: 104-108
Most Viewed
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215
-
Prospective Coronavirus Liver Effects: Available KnowledgeAvishek Mandal*. Prospective Coronavirus Liver Effects: Available Knowledge. Ann Clin Gastroenterol Hepatol. 2023 doi: 10.29328/journal.acgh.1001039; 7: 001-010
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."