Abstract
Research Article
Role of plants, environmental toxins and physical neurotoxicological factors in Amyotrophic lateral sclerosis, Alzheimer Disease and other Neurodegenerative Diseases
Mauro Luisetto*, Naseer Almukhtar, Ahmed Yesvi Rafa, Behzad Nili Ahmadabadi, Ghulam Rasool Mashori, Farhan Ahmad Khan, Ram Kumar Sahu, Gamal Abdul Hamid and Khaled Edbey
Published: 04 March, 2019 | Volume 3 - Issue 1 | Pages: 001-086
Aim of this work is to verify the effect of some neurotoxins, physical factors and geography in presentation of some Relevant Neurological disorder like some form of ASL, PD, AD.
The geographic diffusion of the ASL/PD in west pacific (GUAM foci), and mutation of SOD 1 and other mutations are interesting facts to verify the recent literature about the neurotoxic process.
Related to the references presented a global conclusion about the pathogenetic progression of some neurological disease will be produced as instrument for new hypothesis and for the introduction of new innovative therapeutic strategies.
Read Full Article HTML DOI: 10.29328/journal.jnnd.1001019 Cite this Article Read Full Article PDF
Keywords:
Als; Pd; Ad; Plants neurotoxins; Intracellular neuronal inclusion; Oxidative damages; Epidemiology; Sports; Pesticides; Cianobacteria; Electromagnetic field; Computational biology
References
- Escobar-Khondiker M, Höllerhage M, Muriel MP, Champy P, Bach A, et al. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci. 2007; 27: 7827-7837. Ref.: https://goo.gl/3cFDm8
- N Bonneau, J Le Ven, I Schmitz-Afonso, V Guérineau, I Bajin ba Ndob, et al. Annonaceous acetogenins as environmental neurotoxins: Human exposure from edible Annona fruits. Planta Med. 2012; 78 - PH25. Ref.: https://goo.gl/fdZdir
- Lannuzel A, Michel PP, Caparros-Lefebvre D, Abaul J, Hocquemiller R, et al. Toxicity of Annonaceae for dopaminergic neurons: Potential role in atypical parkinsonism in Guadeloupe. Mov Disord. 2002;17: 84-90. Ref.: https://goo.gl/NhQaGs
- Höllerhage M, Rösler TW, Berjas M, Luo R, Tran K, et al. Neurotoxicity of Dietary Supplements from Annonaceae Species. Int J Toxicol. 2015; 34: 543-550. Ref.: https://goo.gl/3Dn27B
- Zafra-Polo MC, González MC, Estornell E, Sahpaz S, Cortes D. Acetogenins from Annonaceae, inhibitors of mitochondrial complex I. Phytochemistry. 1996; 42: 253-271. Ref.: https://goo.gl/Sdmqi2
- N'Gouemo, B. Koudogbo, H. Pambou Tchivounda, C. Akono‐Nguema, Minko M. Etoua
- Effects of ethanol extract of Annona muricata on pentylenetetrazol‐induced convulsive seizures in mice. Short Communication. 1998. Ref.: https://goo.gl/Dgr8fR
- Moghadamtousi SZ, Rouhollahi E, Hajrezaie M, Karimian H, Abdulla MA, et al. Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int J Surg. 2015; 18: 110-117. Ref.: https://goo.gl/S8fkmi
- Chen Y, Chen JW, Zhai JH, Wang Y, Wang SL, et al. Antitumor activity and toxicity relationship of annonaceous acetogenins. Food Chem Toxicol. 2013; 58: 394-400. Ref.: https://goo.gl/7jsbTN
- Ribeiro Ldo P, Zanardi OZ, Vendramim JD, Yamamoto PT. Comparative toxicity of an acetogenin-based extract and commercial pesticides against citrus red mite. Exp Appl Acarol. 2014; 64: 87-98. Ref.: https://goo.gl/vnpnc7
- Levine RA, Richards KM, Tran K, Luo R, Thomas AL, et al. Determination of Neurotoxic Acetogenins in Pawpaw (Asimina triloba) Fruit by LC-HRMS. J Agric Food Chem. 2015; 63: 1053-1056. Ref.: https://goo.gl/WBmX6q
- Jiang Z, Wang W, Perry G, Zhu X, Wang X. Mitochondrial dynamic abnormalities in amyotrophic lateral sclerosis. Transl Neurodegener. 2015; 4: 14. Ref.: https://goo.gl/vxJowD
- Ravindranath V. Neurolathyrism: mitochondrial dysfunction in excitotoxicity mediated by L-beta-oxalyl aminoalanine. Neurochem Int. 2002; 40: 505-509. Ref.: https://goo.gl/AueXgQ
- Potts LF, Luzzio FA, Smith SC, Hetman M, Champy P, et al. Annonacin in Asimina triloba fruit: Implication for neurotoxicity. Neurotoxicology. 2012; 33: 53-58. Ref.: https://goo.gl/qi3SpF
- Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, et al. Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem. 2005; 280: 42026-42035. Ref.: https://goo.gl/yYvYvH
- Shen WB, McDowell KA, Siebert AA, Clark SM, Dugger NV, et al. Environmental neurotoxin-induced progressive model of parkinsonism in rats. Ann Neurol. 2010; 68: 70-80. Ref.: https://goo.gl/3M1CtV
- Scott LL, Downing TG. B-N-Methylamino-l-alanine (BMAA) Toxicity Is Gender and Exposure-Age Dependent in Rats. Toxins (Basel). 2018; 10: 16. Ref.: https://goo.gl/69V7GM
- Uversky VN. Neurotoxicant-induced animal models of Parkinson's disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004; 318: 225-241. Ref.: https://goo.gl/3Erqvj
- de Pedro N, Cautain B, Melguizo A, Vicente F, Genilloud O, Peláez F, et al. Mitochondrial complex I inhibitors, acetogenins, induce HepG2 cell death through the induction of the complete apoptotic mitochondrial pathway. J Bioenerg Biomembr. 2013; 45: 153-164. Ref.: https://goo.gl/YqcVof
- Degli Esposti M, Ghelli A, Ratta M, Cortes D, Estornell E. Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). Biochem J. 1994; 301: 161-167. Ref.: https://goo.gl/gqXFpE
- Segura Aguilar J, Kostrzewa RM. Neurotoxins and neurotoxic species implicated in neurodegeneration. Neurotox Res. 2004; 6: 615-630. Ref.: https://goo.gl/h1K7Sc
- Caparros-Lefebvre D, Steele J, Kotake Y, Ohta S. Geographic isolates of atypical Parkinsonism and tauopathy in the tropics: possible synergy of neurotoxins. Mov Disord. 2006; 21: 1769-1771. Ref.: https://goo.gl/twX6pZ
- Khandare AL, Kumar RH, Meshram II, Arlappa N, Laxmaiah A, et al. Current scenario of consumption of Lathyrus sativus and lathyrism in three districts of Chhattisgarh State, India. Toxicon. 2018; 150: 228-234. Ref.: https://goo.gl/Kf6kGC
- Salama M, Arias-Carrión O. Natural toxins implicated in the development of Parkinson’s disease. Ther Adv Neurol Disord. 2011; 4: 361-373. Ref.: https://goo.gl/fo1KA7
- Bozzoni V, Pansarasa O, Diamanti L, Nosari G, Cereda C. Amyotrophic lateral sclerosis and environmental factors. Funct Neurol. 2016; 31: 7-19. Ref.: https://goo.gl/UpPN7K
- Pan-Montojo F, Reichmann H. Considerations on the role of environmental toxins in idiopathic Parkinson’s disease pathophysiology. Transl Neurodegener. 2014; 3: 10. Ref.: https://goo.gl/EJ9URY
- Rafael H, David JO, Vilca AS. Etiology and treatment of amyotrophic lateral sclerosis. Am J Neurodegener Dis. 2017; 6: 1-8. Ref.: https://goo.gl/rtZ4Ra
- Das K, Nag C, Ghosh M. Familial, Environmental, and Occupational Risk Factors in Development of Amyotrophic Lateral Sclerosis. N Am J Med Sci. 2012; 4: 350-355. Ref.: https://goo.gl/s1hzKe
- Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta-analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res. 2012; 117: 112-119. Ref.: https://goo.gl/WU5nH4
- Zhou H, Chen G, Chen C, Yu Y, Xu Z. Association between extremely low-frequency electromagnetic fields occupations and amyotrophic lateral sclerosis: a meta-analysis. PLoS One. 2012; 7: e48354. Ref.: https://goo.gl/HfDCt4
- Banack SA, Cox PA. Biomagnification of cycad neurotoxins in flying foxes: implications for ALS-PDC in Guam. Neurology. 2003; 61: 387-389. Ref.: https://goo.gl/Y6kXf2
- Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci U S A. 2003; 100: 13380-13383. Ref.: https://goo.gl/Xm4YVK
- Ingre C, Roos PM, Piehl F, Kamel F, Fang F. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol. 2015; 7: 181-193. Ref.: https://goo.gl/Lgk4yL
- Deng HX, Zhai H, Bigio EH, Yan J, Fecto F, et al. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol. 2010; 67: 739-748. Ref.: https://goo.gl/v2VJqB
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004; 10: S10-S17. Ref.: https://goo.gl/JoNjK7
- Huss A, Spoerri A, Egger M, Kromhout H, Vermeulen R, et al. Occupational exposure to magnetic fields and electric shocks and risk of ALS: the Swiss National Cohort. Amyotroph Lateral Scler Frontotemporal Degener. 2015; 16: 80-85. Ref.: https://goo.gl/W5AeYm
- Martin S, Al Khleifat A, Al-Chalabi A. What causes amyotrophic lateral sclerosis? 2017; 6: 371. Ref.: https://goo.gl/KJTgiH
- Abhinav K, Al-Chalabi A, Hortobagyi T, Leigh PN. Electrical injury and amyotrophic lateral sclerosis: a systematic review of the literature. J Neurol Neurosurg Psychiatry. 2007; 78: 450-453. Ref.: https://goo.gl/8TSPgf
- Delzor A, Couratier P, Boumédiène F, Nicol M, Druet-Cabanac M, et al. Searching for a link between the L-BMAA neurotoxin and amyotrophic lateral sclerosis: a study protocol of the French BMAALS programme. BMJ Open. 2014; 4: e005528. Ref.: https://goo.gl/mGcWPh
- Henry KA, Fagliano J, Jordan HM, Rechtman L, Kaye WE. Geographic Variation of Amyotrophic Lateral Sclerosis Incidence in New Jersey, 2009–2011. Am J Epidemiol. 2015; 182: 512-519. Ref.: https://goo.gl/mD75DD
- Rooney J, Heverin M, Vajda A, Crampsie A, Tobin K, et al. An Exploratory Spatial Analysis of ALS Incidence in Ireland over 17.5 Years (1995 – July 2013). PLoS One. 2014; 9: e96556. Ref.: https://goo.gl/jCeEyt
- Logroscino G, Piccininni M. Amyotrophic Lateral Sclerosis Descriptive Epidemiology: The Origin of Geographic Difference. Neuroepidemiology. 2019; 52: 93-103. Ref.: https://goo.gl/2hDwXo
- Scott KM, Abhinav K, Stanton BR, Johnston C, Turner MR, et al. Geographical clustering of amyotrophic lateral sclerosis in South-East England: a population study. Neuroepidemiology. 2009; 32: 81-88. Ref.: https://goo.gl/ZrVzPN
- Uenal H, Rosenbohm A, Kufeldt J, Weydt P, Goder K, et al. Incidence and Geographical Variation of Amyotrophic Lateral Sclerosis (ALS) in Southern Germany – Completeness of the ALS Registry Swabia. PLoS One. 2014; 9: e93932. Ref.: https://goo.gl/5hjGV4
- Kokubo Y, Kuzuhara S, Narita Y. Geographical distribution of amyotrophic lateral sclerosis with neurofibrillary tangles in the Kii Peninsula of Japan. J Neurol. 2000; 247: 850-852. Ref.: https://goo.gl/oqGkV8
- Virginia Bozzoni, Orietta Pansarasa, Luca Diamanti, Guido Nosari, Cristina Cereda, et al. Amyotrophic lateral sclerosis and environmental factors. Funct Neurol. 2016; 31: 7-19. Ref.: https://goo.gl/WpMiMG
- Yu Y, Su FC, Callaghan BC, Goutman SA, Batterman SA, et al. Environmental Risk Factors and Amyotrophic Lateral Sclerosis (ALS): A Case-Control Study of ALS in Michigan. PLoS One. 2014; 9: e101186. Ref.: https://goo.gl/USCX5B
- Kuzuhara S. [ALS-parkinsonism-dementia complex of the Kii peninsula of Japan (Muro disease). Historical review, epidemiology and concept]. Rinsho Shinkeigaku. 2007; 47: 962-965. Ref.: https://goo.gl/dGrJ61
- Zarei S, Carr K, Reiley L, Diaz K, Guerra O, et al. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015; 6: 171. Ref.: https://goo.gl/WAWsUw
- Ludolph AC, Hugon J, Dwivedi MP, Schaumburg HH, Spencer PS. Studies on the aetiology and pathogenesis of motor neuron diseases. 1. Lathyrism: clinical findings in established cases. Brain. 1987; 110: 149-465. Ref.: https://goo.gl/Z7Q7KU
- Khandare AL, Babu JJ, Ankulu M, Aparna N, Shirfule A, et al. Grass pea consumption & present scenario of neurolathyrism in Maharashtra State of India. Indian J Med Res. 2014; 140: 96-101. Ref.: https://goo.gl/bW4wgx
- Uccelli R, Binazzi A, Altavista P, Belli S, Comba P, et al. Geographic distribution of amyotrophic lateral sclerosis through motor neuron disease mortality data. Eur J Epidemiol. 2007; 22: 781-790. Ref.: https://goo.gl/548yma
- Schwartz GG, Rundquist BC, Simon IJ, Swartz SE. Geographic distributions of motor neuron disease mortality and well water use in U.S. counties. Amyotroph Lateral Scler Frontotemporal Degener. 2017; 18: 279-283. Ref.: https://goo.gl/uDefbU
- Li W, Lee MH, Henderson L, Tyagi R, Bachani M, et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med. 2015; 7: 307ra153. Ref.: https://goo.gl/FbY7so
- Chio A, Calvo A, Dossena M, Ghiglione P, Mutani R, et al. ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph Lateral Scler. 2009; 10: 205-209. Ref.: https://goo.gl/BdqgVD
- Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005; 128: 472-476. Ref.: https://goo.gl/yz7Ubm
- Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005; 20: 237-242. Ref.: https://goo.gl/r2Zfg3
- Delzor A, Couratier P, Boumédiène F, Nicol M, Druet-Cabanac M, et al. Searching for a link between the L-BMAA neurotoxin and amyotrophic lateral sclerosis: a study protocol of the French BMAALS programme. BMJ Open. 2014; 4: e005528. Ref.: https://goo.gl/RKPkSP
- Manzano GM, Giuliano LM, Nóbrega JA. A brief historical note on the classification of nerve fibers Arq Neuropsiquiatr. 2008; 66: 117-119. Ref.: https://goo.gl/8ZcD7C
- Tapia R. Cellular and molecular mechanisms of motor neuron death in amyotrophic lateral sclerosis: a perspective. Front Cell Neurosci. 2014; 8: 241. Ref.: https://goo.gl/bLTZqR
- Bélanger M, Allaman I, Magistretti PJ. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011; 14: 724-738. Ref.: https://goo.gl/6WLzWp
- Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, et al. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. Int J Mol Sci. 2015; 16: 25959-25981. Ref.: https://goo.gl/smjsmd
- Bertamini M, Marzani B, Guarneri R, Guarneri P, Bigini P, et al. Mitochondrial oxidative metabolism in motor neuron degeneration (mnd) mouse central nervous system. Eur J Neurosci. 2002; 16: 2291-2296. Ref.: https://goo.gl/KVDq6F
- Tefera TW, Borges K. Metabolic Dysfunctions in Amyotrophic Lateral Sclerosis Pathogenesis and Potential Metabolic Treatments. Front Neurosci. 2016; 10: 611. Ref.: https://goo.gl/EhcDe5
- Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005; 20: 237-242. Ref.: https://goo.gl/kMmb95
- Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta-analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res. 2012; 117: 112-119. Ref.: https://goo.gl/bA3Xie
- Alessandro Cristani, Elisa Romagnoli. Storia del rapporto tra sclerosi laterale amiotrofica e sport: alla ricerca dell’etiopatogenesi sconosciuta. Recenti Progressi Medicina. 2006. Ref.: https://goo.gl/33y53X
- Feddermann-Demont N, Junge A, Weber KP, Weller M, Dvořák J, et al. Prevalence of potential sports‐associated risk factors in Swiss amyotrophic lateral sclerosis patients. Brain Behav. 2017; 7: e00630. Ref.: https://goo.gl/ikToDA
- Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BM, et al. Spinal Cord Ischemia: Practical Imaging Tips, Pearls, and Pitfalls. AJNR Am J Neuroradiol. 2015; 36: 825-830. Ref.: https://goo.gl/n8L7zd
- Mauro Luisetto , Behzad N-A, Nilesh MM, Ghulam RM, Ram KS, et al. Amyotrophic Lateral Sclerosis and Endogenous-Esogenous Toxicological Movens: New Model to Verify Other Pharmacological Strategies. Arch Pathol Clin Res. 2018; 2: 28-48. Ref.: https://goo.gl/QpkZd6
- Kim S, Kim H, Kralik JD, Jeong J. Vulnerability-Based Critical Neurons, Synapses, and Pathways in the Caenorhabditis elegans Connectome. PLoS Comput Biol. 2016; 12: e1005084. Ref.: https://goo.gl/HedD1H
- Caller TA, Chipman JW, Field NC, Stommel EW. Spatial analysis of amyotrophic lateral sclerosis in Northern New England, USA, 1997-2009. Muscle Nerve. 2013; 48: 235-241. Ref.: https://goo.gl/cSWyYs
- Lee BC, Johng HM, Lim JK, Jeong JH, Baik KY, et al. Effects of extremely low frequency magnetic field on the antioxidant defense system in mouse brain: a chemiluminescence study. J Photochem Photobiol B. 2004; 73: 43-48. Ref.: https://goo.gl/bsRTqj
- Piazza O, Sirén AL, Ehrenreich H. Soccer, neurotrauma and amyotrophic lateral sclerosis: is there a connection? Curr Med Res Opin. 2004; 20: 505-508. Ref.: https://goo.gl/X5g9Zd
- Scotter EL, Chen HJ, Shaw CE. TDP-43 Proteinopathy and ALS: Insights into Disease Mechanisms and Therapeutic Targets. Neurotherapeutics. 2015; 12: 352-363. Ref.: https://goo.gl/NkJD6Q
- Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, et al. Protein misfolding in neurodegenerative diseases: implications and strategie. Transl Neurodegener. 2017; 6: 6. Ref.: https://goo.gl/4RdYnD
Figures:

Figure 1
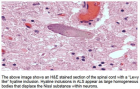
Figure 2
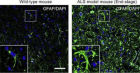
Figure 3
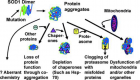
Figure 4
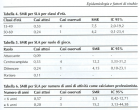
Figure 5
Figure 6
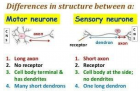
Figure 7
Figure 8
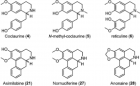
Figure 9
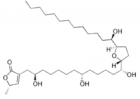
Figure 10

Figure 11
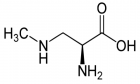
Figure 12

Figure 13

Figure 14

Figure 15
Figure 16
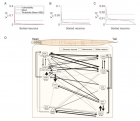
Figure 17
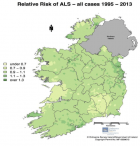
Figure 18
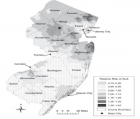
Figure 19
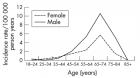
Figure 20
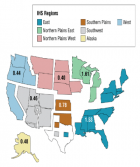
Figure 21
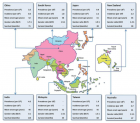
Figure 22
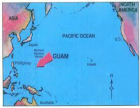
Figure 23
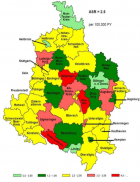
Figure 24
Figure 25
Similar Articles
-
Analysis of early Versus Delayed Carotid Surgery after Acute Ischemic StrokePEROU Sébastien*,DETANTE Olivier,SPEAR Rafaelle,PIRVU Augustin,ELIE Amandine,MAGNE Jean-Luc. Analysis of early Versus Delayed Carotid Surgery after Acute Ischemic Stroke. . 2017 doi: 10.29328/journal.jnnd.1001001; 1: 001-011
-
Protective functions of AEURA in Cell Based Model of Stroke and Alzheimer diseaseJigar Modi,Ahmed Altamimi,Ashleigh Morrell,Hongyuan Chou,Janet Menzie,Andrew Weiss,Michael L Marshall, Andrew Li,Howard Prentice*,Jang-Yen Wu*. Protective functions of AEURA in Cell Based Model of Stroke and Alzheimer disease. . 2017 doi: 10.29328/journal.jnnd.1001003; 1: 016-023
-
Lateralized Cerebral Amyloid Angiopathy presenting with recurrent Lacunar Ischemic StrokeYi Li*, Ayman Al-Salaimeh,Elizabeth DeGrush,Majaz Moonis*. Lateralized Cerebral Amyloid Angiopathy presenting with recurrent Lacunar Ischemic Stroke. . 2017 doi: 10.29328/journal.jnnd.1001005; 1: 029-032
-
Experimental ‘hindbrain related’ syringomyelia: some mechanisms of spinal cord damageSergey N Larionov*,Sorokovikov VA,Rudakova AV. Experimental ‘hindbrain related’ syringomyelia: some mechanisms of spinal cord damage. . 2017 doi: 10.29328/journal.jnnd.1001006; 1: 033-038
-
Comorbidity of alcohol dependence with attention-deficit/hyperactivity disorder and the role of executive dysfunctionsCaterina Pistarini*,Gloria Tosi,Giovanni Vittadini,Ines Giorgi,Elena Fiabane,Paola Palladino. Comorbidity of alcohol dependence with attention-deficit/hyperactivity disorder and the role of executive dysfunctions . . 2018 doi: 10.29328/journal.jnnd.1001008; 2: 001-010
-
Spinal muscular atrophy counteracted by Agrin biological NT-1654Jes Paul*. Spinal muscular atrophy counteracted by Agrin biological NT-1654. . 2018 doi: 10.29328/journal.jnnd.1001009; 2: 011-013
-
Herpes simplex virus (HSV)-1 encephalitis can induce chronic anti-N-methyl-D-aspartate receptor (NMDAR) encephalitisSusanne Buechner*,Gabriele J Sixt,Igor Florio. Herpes simplex virus (HSV)-1 encephalitis can induce chronic anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. . 2018 doi: 10.29328/journal.jnnd.1001012; 2: 033-038
-
Neurobiology of Common Sleep DisordersZhao Liu*,Abdullah Tolaymat,Sreenivas K Avula. Neurobiology of Common Sleep Disorders. . 2018 doi: 10.29328/journal.jnnd.1001013; 2: 039-046
-
Vigour of CRISPR/Cas9 Gene Editing in Alzheimer’s DiseaseJes Paul*. Vigour of CRISPR/Cas9 Gene Editing in Alzheimer’s Disease. . 2018 doi: 10.29328/journal.jnnd.1001014; 2: 047-051
-
Cranioplasty with preoperatively customized Polymethyl-methacrylate by using 3-Dimensional Printed Polyethylene Terephthalate Glycol MoldMehmet Beşir Sürme*,Omer Batu Hergunsel,Bekir Akgun,Metin Kaplan. Cranioplasty with preoperatively customized Polymethyl-methacrylate by using 3-Dimensional Printed Polyethylene Terephthalate Glycol Mold. . 2018 doi: 10.29328/journal.jnnd.1001016; 2: 052-064
Recently Viewed
-
Depression as a civilization-deformed adaptation and defence mechanismBohdan Wasilewski*,Olha Yourtsenyuk,Eugene Egan. Depression as a civilization-deformed adaptation and defence mechanism. Insights Depress Anxiety. 2020: doi: 10.29328/journal.ida.1001013; 4: 008-011
-
Drinking-water Quality Assessment in Selective Schools from the Mount LebanonWalaa Diab, Mona Farhat, Marwa Rammal, Chaden Moussa Haidar*, Ali Yaacoub, Alaa Hamzeh. Drinking-water Quality Assessment in Selective Schools from the Mount Lebanon. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001061; 8: 018-024
-
Rapid Microbial Growth in Reusable Drinking Water BottlesQishan Liu*,Hongjun Liu. Rapid Microbial Growth in Reusable Drinking Water Bottles. Ann Civil Environ Eng. 2017: doi: 10.29328/journal.acee.1001007; 1: 055-062
-
Beneficial effects of a ketogenic diet in a woman with Charcot-Marie-Tooth diseaseElvira Rostanzo,Anna Maria Aloisi*. Beneficial effects of a ketogenic diet in a woman with Charcot-Marie-Tooth disease. Arch Food Nutr Sci. 2022: doi: 10.29328/journal.afns.1001040; 6: 068-072
-
Isolation and Influence of Carbon Source on the Production of Extracellular Polymeric Substance by Bacteria for the Bioremediation of Heavy Metals in Santo Amaro CityLeila Thaise Santana de Oliveira Santos*, Kayque Frota Sampaio, Elisa Esposito, Elinalva Maciel Paulo, Aristóteles Góes-Neto, Amanda da Silva Souza, Taise Bomfim de Jesus. Isolation and Influence of Carbon Source on the Production of Extracellular Polymeric Substance by Bacteria for the Bioremediation of Heavy Metals in Santo Amaro City. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001060; 8: 012-017
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."